1. Background
SARS-CoV-2 was declared a pandemic after the first case was identified in China and swiftly spread globally in December 2019 (1). As of October 14, 2022, WHO has reported over 620 million confirmed cases worldwide, resulting in over 6.5 million deaths (2). COVID-19 manifestations vary from asymptomatic or moderate symptoms to severe sickness and death. Common symptoms include fever, cough, and shortness of breath. Symptoms may manifest between 2 and 14 days following exposure to the virus (3). Nearly 80% of COVID-19 infections are mild or asymptomatic, 15% are severe (need oxygen supplementation), and 5% are life-threatening and require mechanical ventilation (4).
COVID-19 may disproportionately impact the elderly and those with cardiologic, pulmonary, renal, and metabolic comorbidities (5). This is also manifest in pregnancies where it is a well-documented fact that pregnancy-related alterations in maternal physiology make pregnant women more susceptible to a more severe course of pneumonia, resulting in higher maternal and fetal morbidity and mortality (6, 7). This is because the maternal respiratory system undergoes various physiological alterations throughout pregnancy. These alterations expose the mother to hypoxia, which the body reacts to by increasing tidal volume and hyperventilating. Hyperventilation allows pregnant women to breathe in more air, which in turn makes pregnant women more susceptible to contracting airborne SARS-CoV-2 than ordinary people; moreover, progesterone-mediated alterations in nasal mucosa throughout pregnancy may cause the virus to adhere to the upper respiratory system, which in itself makes it difficult to eradicate (8).
Although vertical transmission of SARS-CoV-2 is rare, it appears to be found in a minority of third-trimester COVID-19 pregnant women (9). This may be because COVID-19 increases the rate of feto-maternal outcomes, including preterm birth, premature rupture of membranes, preterm premature rupture of membranes, gestational diabetes mellitus, gestational hypertension, preeclampsia, eclampsia, fetal distress, stillbirth, intrauterine fetal demise, postpartum hemorrhage, maternal mortality, and prolonged labor. Neonatal outcomes (which may be associated with COVID-19) are iatrogenic preterm birth and neonatal intensive care unit (NICU) admission. In addition, pneumonia during pregnancy increases the risk of preterm delivery, low birth weight, and small for gestational age infants (10). It is of note that the literature shows no indication of cesarean birth as not being beneficial in reducing potential vertical transmission from a pregnant woman with COVID-19 to vaginal delivery. The route of delivery depends on the severity of the illness and the obstetric indications (11).
2. Objectives
In the present study, the researchers investigated clinical manifestations and collected data based on radiologic findings, indications for cesarean delivery, underlying conditions, and critical outcome of mothers and newborns in women with terminated pregnancies infected with COVID-19 at the time of cesarean delivery.
3. Methods
3.1. Study Population
This observational cross-sectional study was conducted as an investigation of all pregnant patients with positive SARS-CoV-2 molecular test results who delivered their neonates via cesarean section at Razi Hospital, Ahvaz Jundishapur University of Medical Sciences between March 20, 2020, and March 21, 2021. Inclusion criteria were proof of pregnancy, positive SARS-CoV-2 molecular test, existence of complete medical records, proof of cesarean delivery, and written informed consent. Exclusion criteria were unwillingness to participate in the study and lack of or incomplete medical records.
3.2. Demographic Characteristics
Medical records were used to obtain descriptive data regarding fetal and maternal COVID-19 disease severity. These data were subsequently subcategorized into demographic, clinical, and obstetrical data. Demographic data included age, educational level, occupation, smoking, alcohol, or substance abuse. Clinical data included past medical history (including diabetes mellitus, hypertension, previous cardiac or pulmonary disease, thalassemia, hemophilia, and immune deficiency) and physical examination at the time of hospitalization. Obstetrics data included gravidity, parity, abortion, last menstrual period, gestational age, and fetal lie. The researchers then evaluated the maternal and fetal COVID-19 disease severity using different parameters. Maternal parameters included signs, symptoms, body mass index (BMI), family involvement, oxygen saturation (SpO2) before and after cesarean delivery, ICU admission, mechanical ventilation, hemorrhage, and death. Fetal parameters included birth weight, neonatal SARS-CoV-2 molecular test, Apgar score, NICU admission, and death.
3.3. Statistical Analysis
All analyses were conducted using SPSS version 24 (SPSS Inc, Chicago, IL, USA). The researchers calculated the mean and SD for continuous variables and the number and percentage for categorical variables.
4. Results
4.1. Chronological and Gestational Age
Ninety-eight pregnant women with positive SARS-CoV-2 molecular tests were admitted during the COVID-19 pandemic and underwent cesarean delivery. Of the 98 pregnant women, 20 (20.4%) were less than 25 years, 17 (17.3%) were 26 - 30 years, 35 (35.7%) were 31 - 35 years, and 26 (26.5%) were more than 35 years. The mean age of the population was 31.31 ± 7.16 years. The mean gestational age for the population was 36.45 ± 3.334 weeks. Complete redundancy for gestational age can be found in Table 1.
| Gestational Age | Frequency (%) |
|---|---|
| Less than 35 weeks | 26 (26.53) |
| 36 - 39 weeks | 60 (61.22) |
| 40 weeks and more | 12 (12.24) |
| Total | 98 (100) |
The Frequency and Percentage of Gestational Age
4.2. Cesarean Delivery Indications and Mode of Anesthesia
The most prevalent indication for cesarean delivery was fetal distress (28.6%), followed by preeclampsia (21.4%). Other indications are listed in Table 2. Ninety-four (95.9%) cesarean deliveries were carried out emergently, and 4 (4.1%) were carried out as elective procedures. Ninety-four (95.9%) pregnant women underwent cesarean delivery by spinal anesthesia.
| Cesarean Indication | Frequency (%) |
|---|---|
| Fetal distress | 28 (28.6) |
| Preeclampsia and low bishop score | 21 (21.4) |
| Meconium | 16 (16.3) |
| Previous cesarean history | 9 (9.2) |
| Cesarean near death | 3 (3.1) |
| Other indications (placental abruption, myomectomy, umbilical cord prolapse, etc.) | 21 (21.4) |
| Total | 98 (100) |
The Frequency and Percentage of Cesarean Indication
4.3. Symptoms
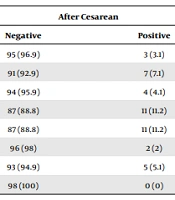
Cough (46.9%) and dyspnea (42.9%) were the most common symptoms before cesarean delivery, whereas fever (11.2%) and vomiting/diarrhea (11.2%) were the prevalent symptoms after cesarean delivery. Other symptoms are listed in Table 3.
| Maternal Symptoms | Before Cesarean | After Cesarean | ||
|---|---|---|---|---|
| Negative | Positive | Negative | Positive | |
| Cough | 52 (53.1) | 46 (46.9) | 95 (96.9) | 3 (3.1) |
| Dyspnea | 56 (57.1) | 42 (42.9 | 91 (92.9) | 7 (7.1) |
| Myalgia | 67 (68.4) | 31 (31.6) | 94 (95.9) | 4 (4.1) |
| Fever | 70 (71.4) | 28 (28.6) | 87 (88.8) | 11 (11.2) |
| Vomiting/diarrhea | 96 (98) | 2 (2) | 87 (88.8) | 11 (11.2) |
| Fatigue | 75 (76.5) | 23 (23.5) | 96 (98) | 2 (2) |
| Sore throat | 94 (95.9) | 4 (4.1) | 93 (94.9) | 5 (5.1) |
| Other symptoms | 83 (84.7) | 15 (15.3) | 98 (100) | 0 (0) |
The Frequency and Percentage of Maternal Symptoms Before and After Cesarean Delivery a
4.4. Chest High-Resolution Computed Tomography
Chest high-resolution computed tomography (HRCT) was used to investigate whether pulmonary involvement was present. The HRCT was not administered for 11 (11.22%) pregnant women. Lung involvement was not seen in 38 (38.77%) pregnant women’s HRCT. The results of HRCT showed that 35 (35.71%) pregnant women suffered from mild involvement, 8 (8.16%) suffered from moderate involvement, and 6 (6.12%) pregnant women suffered from severe COVID-19 pulmonary involvement.
4.5. Underlying Conditions
The most prevalent underlying condition was hypothyroidism (11.2%), followed by diabetes mellitus (9.2%). Hypertension (8.2%) and asthma (6.1%) were also seen in the population. The underlying condition was not observed in 59 (60.2%) pregnant women’s medical records. Family involvement was observed in 35 (35.71%) cases, whereas it did not occur for 63 (64.28%) pregnant women. The mean BMI was 30.22 ± 5.52 kg/m2, and the median was 29.5 kg/m2 for the population. Only one subject had a history of smoking.
4.6. Diagnoses on Admission
Forty-three (43.9%) pregnant women were admitted due to COVID-19, and 55 (56.1%) were admitted due to obstetrics indications other than COVID-19. Complete diagnosis records are listed in Table 4.
| Primary Diagnoses | Frequency (%) |
|---|---|
| COVID-19 | 43 (43.9) |
| Rupture of membranes | 14 (14.28) |
| Labor pain + COVID-19 | 13 (13.26) |
| Preterm labor | 9 (9.18) |
| Post-term pregnancy | 9 (9.18) |
| Others | 10 (10.2) |
| Total | 98 (100) |
The Frequency and Percentage of Primary Diagnoses
4.7. Vital Signs and SpO2
The mean SpO2 before cesarean delivery was 97.69 ± 1.7, whereas this measure was 95.59 ± 1.45 after cesarean delivery. The mean temperature was 36.78 ± 0.338°C before and 36.72 ± 0.383°C after cesarean delivery. The mean respiratory rate was 18.77 ± 3.609 before cesarean delivery and 17.09 ± 2.957 after this procedure.
4.8. Critical Outcomes
Thirteen (13.26%) pregnant women were admitted to the ICU during this period, of whom 5 (5.1%) were intubated and needed mechanical ventilation. The mean hospitalization period was 5.21 ± 4.584 days. Three (3%) pregnant women suffered from hemorrhage, all categorized as postpartum hemorrhage. Only 1 (1%) pregnant woman developed a wound infection after the procedure. Five (5.1%) pregnant women died due to COVID-19 complications during this period.
4.9. Neonatal Outcomes
The Apgar score was 0 - 1 for 1 neonate, 2 - 4 for 1 neonate, 8 - 9 for 4 neonates, and 9 - 10 for 98 neonates, of whom 14 (14.3%) were admitted to NICU, and 2 (2%) died. All the neonates were tested for SARS-CoV-2 24 hours after birth, of which all the tests were negative; vertical transmission did not occur. The birth weight was less than 2500 g for 10 (10.2%) neonates, 2500 - 3500 g for 73 (74.5%) neonates, and 3500 g for 15 (15.3%) neonates. The mean birth weight was 3097 g.
5. Discussion
As COVID-19 continues to spread worldwide, there will likely be an increase in infections among pregnant women worldwide. Accordingly, it is essential that pregnant women and their families, as well as the general public and health care providers, receive the most accurate information available (12). High rates of preterm birth and cesarean delivery have been reported in women with SARS-CoV-2 infection. However, studies have insufficient power to evaluate rare events such as stillbirth (13). In this study, most of the pregnant women were in the third trimester of their pregnancy. This demonstrates the possible increased risk of being symptomatic or morbidity for those infected with SARS-CoV-2, which shows the importance of monitoring pregnant women in the third trimester.
Higher incidences of COVID-19 involvement in the third trimester of pregnancy have also been seen in previous studies (14-16). Cesarean delivery in 26.5% of the studied population was carried out before 35 weeks of gestational age. However, the global rate of preterm birth is 11% (17). Rusconi et al. stated that the rate of preterm birth decreased following the implementation of COVID-19 restriction policies (18). The higher rate of preterm birth may be because the researchers only studied COVID-19-infected women who underwent cesarean delivery.
According to HRCT findings, 6% of the population had severe pneumonia. The same findings were seen in a systematic review and meta-analysis by Mirbeyk et al., in which 37 articles and 364 pregnant women were analyzed (19). In this study, cough and dyspnea are the most frequent symptoms among pregnant women. Similar findings were seen in the study by Sekkarie et al. conducted on 21 848 pregnant women, in which the most frequent symptoms were cough and dyspnea (20). The mean BMI was 30.22 ± 5.52 kg/m2 for the studied population, indicating that most women suffer from obesity. This implies that obesity might be a risk factor for COVID-19 infection and its morbidity. Previous investigations also demonstrate that obesity is the most prevalent comorbidity among women suffering from COVID-19 (16, 21). Thirty-five pregnant women (35.7%) had a history of close contact with SARS-CoV-2–positive family members. This issue was also mentioned by Tabatabai et al. (22), emphasizing the importance of educating pregnant women about this disease.
In this study, most of the cesarean deliveries (95.9%) were carried out emergently, in which the most prevalent indication was fetal distress (28.6%), followed by preeclampsia (21.4%). As mentioned before, the route of delivery depends on the severity of the illness and the obstetric indications (11). In a systematic review, Yang et al. evaluated 18 studies consisting of 114 pregnant women, showing that the most prevalent indications for cesarean delivery were preeclampsia and fetal distress (23). In this study, the researchers investigated the underlying conditions in SARS-CoV-2-positive pregnant women undergoing cesarean delivery, of which the most prevalent comorbidity was hypothyroidism (11.2%), followed by diabetes mellitus (9.2%). However, 60.2% of the population did not have any underlying condition. Antsaklis et al. reported that hypothyroidism is one of the prevalent comorbidities among pregnant women (24). Kurian et al. found that diabetes in pregnant women is substantially related to COVID-19 infection severity and has adverse outcomes for mothers and newborns (25).
There was only 1 case of wound infection in our population. Antonello et al. concluded that the incidence of surgical site infection decreased during the COVID-19 period, which may result from safer precautions adopted by healthcare professionals (26). We found that 3 (3%) women suffered from postpartum hemorrhage. Based on a score-matched analysis by Januszewski et al., the number of packed red blood cell (RBC) units transfused, the prevalence of postpartum hemorrhage, and the predicted blood loss all elevated after COVID-19 deliveries, which is perceived as another threat for SARS-CoV-2–positive pregnant women (27). Thirteen women (13.26%) were admitted to the ICU, of whom 5.1% were intubated at the time of hospitalization. Based on the study by Wang et al., COVID-19 could be a risk factor for ICU admission and intubation (28). The maternal mortality rate was 5.1% in our population, which was higher than in previous studies (29, 30). The higher mortality rate may be due to the increased number of studied patients in the aforementioned studies and the fact that the current study investigated women who underwent cesarean delivery.
The Apgar score was 9 - 10 for 92 (93.9%) neonates and less than 7 for 6 (6.1%) neonates. Based on a systematic review and meta-analysis by de Medeiros et al., which included 70 studies and 10 047 pregnant women in the third trimester, the incidence of Apgar < 7 was 19%. The higher rate may be due to the higher number of subjects in this systematic review (31). The NICU admission rate was 14.3%, which was lower than the study by Ward et al. (32). This could be attributed to the higher number of patients in the aforementioned study and the fact that the neonatal mortality rate was 2% in this study. This rate is in line with a previous study by Marchand et al. (29).
The birth weight of 10.2% of neonates was less than 2500 g, which is considered low birth weight in the literature. The incidence was lower than global estimates by WHO; however, a previous study by de Medeiros et al. highlighted that COVID-19 could increase low birth weight incidence (31). The difference could be due to the limited number of pregnant women and neonates. The limitation imposed on the current study was the small sample size. The researchers highly recommend studying cesarean delivery in pregnant women suffering from COVID-19 in a larger population. Moreover, this study did not analyze the neonatal cord, placenta, amniotic fluid, urine, and fecal and rectal samples. These samples could provide better insight into vertical transmission in the future. Another limitation of the current study is the limited demographic data, which could aid researchers in evaluating the population more specifically.
5.1. Conclusions
The majority of our population was in the third trimester of pregnancy at the time of delivery. This in itself implies the need for greater attention and education for mothers in this period. This study also indicates that BMI and obesity are strongly associated with COVID-19 severity. Furthermore, healthcare workers should pay attention to underlying diseases during pregnancy. Due to the COVID-19 morbidity and mortality in pregnant women and neonates, it is highly recommended that a complete team consisting of a gynecologist, obstetrician, neonatologist, anesthesiologist, and internist should be present in the operating theatre during cesarean deliveries.