1. Background
The emergence of resistant bacteria has rapidly increased worldwide, endangering the effectiveness of antibiotics (1, 2). The lack of the antimicrobial function of conventional antibiotics against healthcare-associated pathogens has increased the deaths caused by infections (3). Staphylococcus aureus is a gram-positive bacterial strain that causes a wide range of infections, including skin infections, bacteremia, infective endocarditis, and pneumonia. This pathogenic microorganism has been introduced by the World Health Organization (WHO) as one of the most common nosocomial infectious bacteria (4, 5). The increased resistance of S. aureus to the available antibiotics has caused major obstacles to therapeutic measures (6, 7). Patients admitted to the intensive care unit (ICU) have methicillin-resistant S. aureus (MRSA) as the cause of 52.3% of all nosocomial infections (8). Although MRSA has few treatment options, vancomycin is the main candidate antibiotic for treatment. However, frequent and miss use of this antibiotic has developed S. aureus antimicrobial resistance (9, 10).
Antimicrobial peptides (AMPs) have been introduced as a new generation of antibiotics and potent alternatives to conventional antibiotics with fast and effective antimicrobial functions (11, 12). Antimicrobial peptides are polypeptides that contain fewer than 100 amino acids and usually have cationic properties (13). The epithelial and mucosal epithelial surfaces rely on cationic AMPs as the first line of defense against pathogens (14). Antimicrobial peptides are a crucial part of the host’s innate immune system in the fight against and prevention of infections since they exhibit a wide variety of actions against bacteria, protozoa, and fungi (15). Several cellular targets for AMPs have been identified. Most studies have shown that AMPs kill pathogens by directly rupturing their cell membranes (16). The spread of microorganisms that are resistant to AMPs is far more difficult to achieve. In light of their minimal potential for resistance development and a broad spectrum of action, AMPs are increasingly being considered a viable alternative to traditional antibiotics (17).
Protease-3-mediated cleavage of the C-terminus of human cathelicidin (hCAP-18) yields single human cathelicidin AMPs, each composed of 37 amino acids and weighing between 4 and 5 kDa. Neutrophils, mast cells, natural killer (NK) cells, B cells, and epithelial cells are the common builders of this protein (14). LL-37 has been shown to have several immune system-modifying activities in addition to its potent antibacterial activity (18). Additionally, LL-37 encourages cell proliferation and differentiation and speeds up the re-epithelialization process, all of which aid in the healing of wounds (19, 20). This peptide kills bacteria by destroying cell membranes, inhibiting Lipopolysaccharide, and binding to bacterial DNA (21, 22). Rainbow trout (Oncorhynchus mykiss) skin acid extract contains an AMP called oncorhyncin II, which is derived from the C-terminal (carboxyl terminal) of the histone H1 protein. Structurally, it is cationic, amphipathic, and α helical. This peptide has a molecular weight of 7.2 kDa and consists of 69 amino acids (23). This peptide has the potential to be antimicrobial and is less toxic than other AMPs. Like other AMPs, oncorhyncin II has a destructive effect on a variety of gram-positive and gram-negative bacterial membranes. Specifically, it binds to the host membrane, causing the membrane to collapse and destroying the host’s nucleic acid and protein, which results in bacterial death (23, 24).
2. Objectives
This study aimed to produce recombinant LL-37 and oncorhyncin II and investigate their synergistic effects on S. aureus (ATCC25923).
3. Methods
3.1. Materials
Ampicillin, chloramphenicol, vancomycin, nutrient agar (NA), nutrient broth (NB), Mueller Hinton agar (MHA), Mueller Hinton broth (MHB), resazurin sodium salt, and Ni-NTA kit (Qiagen, Alameda, CA, USA) were used in this study. Every one of the other compounds was of analytical quality.
3.2. Bacterial Strain
The antimicrobial activity of peptides was tested against the gram-positive bacterium S. aureus (ATCC25923). As the host for the expression of the recombinant proteins, Escherichia coli BL21 (DE3) was used.
3.3. Expression and Purification of LL-37 and Oncorhyncin II
The gene sequences of LL-37 (UniProt: P49913) and oncorhyncin II (UniProt: P06350) were optimized to express them in E. coli BL21 (DE3) and were synthesized by Biomatik Company (Cambridge, Canada). Then, they were put into the expression vector, pET32a (Novogene, India). To confirm the bacteria carrying recombinant DNA, polymerase chain reaction (PCR) and mini-preparation plasmid were utilized. Competent bacteria were individually infected with the pET32a–LL-37 and pET32a-oncorhyncin II constructs to express the desired genes. Two recombinant colonies, one grown on plates with ampicillin and chloramphenicol (pET32α–LL-37) and the other grown on plates with ampicillin alone (pET32α-oncorhyncin II), were incubated at 37°C in 2-mL NB medium containing 1 μg/mL ampicillin and 1 μg/mL chloramphenicol and 2-mL NB medium containing 1 μg/mL ampicillin, respectively. The next day, 300 μL of each bacterial inoculation overnight culture was added to 200-mL NB (containing 68 μg/mL ampicillin and 68 μg/mL chloramphenicol for LL-37) and (containing 68μg/mL ampicillin for oncorhyncin II) separately and incubated at 220 rpm at 37°C. To induce protein expression, isopropyl thio β-D-galactosidase (IPTG; 1 mM) was added when the optical cell densities reached ~ 0.6 at OD 600 nm.
The cells were collected by centrifugation at 5000 rpm for 20 minutes following a 4-hour incubation period, and the resulting pellets were frozen at - 20°C. The induction result was also confirmed by running the resulting protein on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (18, 25). The recombinant proteins were purified using denatured conditions with 8M urea, followed by Ni-NTA agarose resin affinity chromatography (Qiagen, Alameda, CA, USA). In addition, 12% SDS-PAGE was used for analysis to determine the protein purity of LL-37 and oncorhyncin II. The quality of purified recombinant proteins was evaluated using an absorbance assay at 280 nm (25, 26).
3.4. Refolding Optimization of LL-37 and Oncorhyncin II
The urea destroys the active folding of proteins. Thus, eliminating urea by the dialysis procedure was crucial for peptide refolding recovery. With that in mind, phosphate-buffered saline (PBS)-based exchange buffers with different amino acids (arginine 0.1 M + proline 0.1 M) with optimum pH (pH = 7) and PBS containing amino acids (arginine 0.1 M + proline 0.1 M) at a pH of 8.5 for LL-37 and oncorhyncin II, respectively, were used in conjunction with a ready-to-use dialysis bag having 10-kDa MWCO in the dialysis process (25, 27). The dialysis was performed at 4°C for 24 hours. To improve the efficacy of dialysis, the PBS was changed every 2 hours. Finally, the dialyzed protein was kept at 4°C for further analysis (28).
3.5. Concentration of LL-37 and Oncorhyncin II
A 10-kDa pore-size Amicon centrifugal filter was used to concentrate the refolded recombinant proteins inside the dialyzer tubes (Merck Millipore, Darmstadt, Germany).
3.6. Antimicrobial Activity Assays of LL-37 and Oncorhyncin II
For the preparation of bacterial inoculums, the standard methodology CLSI MO7-A10 was used. Briefly, the selected isolated colonies were grown in MHB and maintained at 37°C until the culture optical density at 600 nm reached 0.1 (1 × 108 cells/mL). The suspensions were then diluted with MHB at a ratio of 1:100 to yield 1 × 106 colony-forming units (CFU)/mL. The minimum inhibitory concentration (MIC) values of the peptides were measured by the micro broth dilution method against S. aureus by Clinical and Laboratory Standards Institute (CLSI) protocol MO7-A10 (29).
The initial addition of 50 μL of MHB to each well of 96-well microplates from column 1:10 and, then, 2-fold serial dilutions of the dialyzed recombinant proteins LL-37 (245 μg/mL) or oncorhyncin II (767 μg/mL) were performed by adding 50 μL of each protein to the well. Next, 50 μL of prepared bacterial inoculum (106 CFU/mL) was added to each well. Column 11 served as the sterility control and contained 100 μL of MHB, while column 12 contained 100 μL of bacterial inoculum and served as a positive growth control. After 24 hours of incubation at 37°C, 20 μL of resazurin dyes (0.02% w/v) was added to each well, and the plates were incubated for another 2 hours. The concentration of each treatment group in the last blue-colored well was deemed to be the MIC value (30).
The minimum inhibitory concentration for vancomycin against S. aureus was determined by broth micro-dilution according to the CLSI (31). On MHA, 100 μL of microplate blue wells corresponding to the MIC value and the blue wells above MIC values of LL-37 and oncorhyncin II were cultivated to determine the Minimum Bactericidal Concentration (MBC). After 24 hours of incubation at 37°C, the MBC was defined as the lowest concentration of an antimicrobial agent that kills 99.9% of a certain organism. The antibacterial activity was characterized using the MBC/MIC ratio (MBC/MIC = 1 or 2 bactericidal, MBC/MIC = 4 or 16 bacteriostatic (32).
3.7. Synergy Investigations by Checkerboard Technique
Checkerboard experiments were performed to demonstrate the enhanced activity of target recombinant peptides. The tests were conducted using 96-well micro-titer plates with LL-37 and oncorhyncin II at successive concentrations of 2-fold. All plates were prepared with decreasing concentrations of LL-37 in the vertical wells and oncorhyncin II in the horizontal wells. In addition, 50 μL of final inoculum of bacterial suspensions containing 1 × 106 CFU/mL cells were added to the wells, and the plates were incubated at37°C for 24 hours. The synergy interactions were evaluated by determining the fractional inhibitory concentration (FICI) calculated as follows:
FICI = (MIC of drug A combination/MIC drug A alone) + (MIC of drug B combination/MIC drug B alone)
The FIC index (FICI) values were evaluated using the following equation:
FICI = FICA + FICB. The results were defined as follows: ≤ 0.5 = synergistic;
0.5 - 0.75 = partial synergy; 0.76 - 1.0 = additive;1.0 - 4.0 = indifferent; 4.0 = antagonistic (31, 33).
3.8. Time-Kill Assay
The bactericidal kinetics of the recombinant peptides was evaluated by time-kill curves against S. aureus with a starting inoculum of 106 CFU/mL in the exponential phase. LL-37 and oncorhyncin II with 2 × MIC concentrations alone and in combination were applied to investigate their single and combined effects on cell viability. Samples were taken 0, 0.5, 1, 3, 5, 7, 11, and 20 hours after incubation, and the colonies were counted by plating them on MHA. The depletion pattern of viable bacterial cell counts and synergistic effects were calculated after 24 hours of incubation at 37°C (33). The bacterial culture without any additions served as a negative control, whereas vancomycin (1.42 μg/mL) served as a positive control. To be considered antibacterial activity, there must be a decrease in bacteria of ≥ 1 log 10 compared to the original inoculum. In the synergism test, ≥ 2 log 10 and 1 ≤ log 10 ≤ 2 were defined as synergistic and additive, respectively (34). Each test was administered three times.
3.9. Growth Kinetic Assay
To evaluate the activity, bacterial cultures in the mid-log phase (OD 600 = 0.6) were diluted in MHB to achieve an OD 600 of 0.2 (108 CFU/mL). Bacteria were grown in 200-μL volumes in separate culture tubes and treated with 2 × MIC concentrations of LL-37 and oncorhyncin II alone or in combination prior to 37°C incubation. At intervals (0, 1, 3, 5, 7, 11, and 20 hours), the turbidity was measured at 600 nm. The experiment was carried out three times (31). Vancomycin as a positive control (1.42 μg/mL) and untreated bacteria were used as a negative control.
3.10. Statistical Analysis
All data were presented as means and SDs. All results were compared using a 2-way analysis of variance (ANOVA). P-values less than 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism version 9.
4. Results
4.1. Expression, Purification, and Refolding of LL-37 and Oncorhyncin II in Escherichia coli
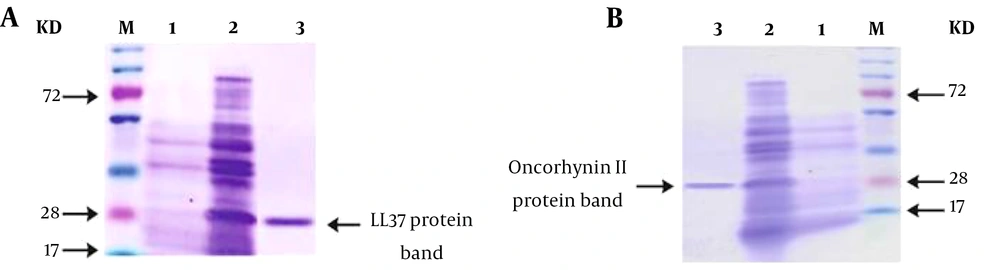
In the expression vector pET32a, the LL-37 and oncorhyncin II proteins were effectively cloned. Afterward, the recombinant pET32a–LL-37 and pET32-oncorhyncin II plasmids were introduced into E. coli BL21 (DE3). Escherichia coli BL21 cells containing recombinant plasmids were treated with IPTG (1 mM) to induce protein expression, and the proteins were purified under denaturing conditions using nickel affinity chromatography (Ni-NTA; Qiagen, Valencia, Spain; Alameda, CA, USA). SDS-PAGE was employed to assess the quality and quantity of purified proteins. The presence of specific bands demonstrated that the LL-37 (28 kDa) and oncorhyncin II (28 kDa) target proteins were effectively expressed (Figure 1).
(A) M, size marker; lane 1, before induction of LL-37; lane 2, expression of LL-37 in Escherichia coli BL21 4 hours after induction; lane 3, purified protein retrieved by nickel affinity chromatography. (B) M, size marker; lane 1, before induction of oncorhyncin II; lane 2, expression of oncorhyncin II in E. coli BL21 4 hours after induction; lane 3, purified protein retrieved by nickel affinity chromatography.
Using a MIC test, the optimal dialysis conditions against S. aureus were confirmed. The results of dialysis in PBS revealed that recombinant proteins LL-37 at pH = 7 and oncorhyncin II at pH = 8.5 and in the present complex of arginine (0.1 M) and proline (0.1 M) amino acids had the highest efficiency. Using a spectrophotometer (Eppendorf, Germany), we determined the concentration of proteins at an OD of 280 nm. After being concentrated using an Amicon centrifugal filter with a pore size of 10 kDa, the resulting proteins had concentrations of 245 μg/mL for LL-37 and 767 μg/mL for oncorhyncin II.
4.2. Antimicrobial Activity Assays and Synergy Studies of LL-37 and Oncorhyncin II
The MIC values were obtained for recombinant LL-37 and oncorhyncin II proteins against S. aureus were 30.6 µg/mL and 47.93 µg/mL, respectively. Also, the MIC of vancomycin was 0.71 μg/mL. Further results of the in vitro antimicrobial activities for the target recombinant peptides against S. aureus are shown in Table 1. To confirm the synergistic activity of LL-37/oncorhyncin II combinations, checkerboard assays were assessed. The results indicated partial synergy between LL-37 and oncorhyncin II on S. aureus, with FICIs of 0.61. The calculated FICI for each peptide and in combination is summarized in Table 1. Based on the results of MBC, each of the LL-37 and oncorhyncin II peptides had a bacteriostatic effect. Also, the MBC values of each peptide alone and in combination with each other are shown in Table 2.
| Microorganism | MIC (µg/mL) | MIC Combination (µg/mL) | Synergism | |||||
|---|---|---|---|---|---|---|---|---|
| LL-37 a | FIC a | Oncorhyncin II b | FIC b | LL-37 c | Oncorhyncin II d | FIC I | ||
| Staphylococcus aureus | 30.6 | 0.12 | 47.93 | 0.49 | 3.82 | 23.96 | 0.61 | Partial synergy |
| Microorganism | MBC (µg/mL) | MBC Combination (µg/mL) | ||
|---|---|---|---|---|
| LL-37 a | Oncorhyncin II a | LL-37 b | Oncorhyncin II b | |
| Staphylococcus aureus | ||||
| MBC | > 122.5 | > 191.75 | > 15.3 | > 95.87 |
| MBC/MIC | > 4 | > 4 | > 4 | > 4 |
| Interpretation | Bacteriostatic | |||
The Results of Minimum Bactericidal Concentration Antimicrobial Peptides LL-37 and Oncorhyncin II Against Staphylococcus aureus (ATCC25923)
4.3. Time-Kill Curves of LL-37 and Oncorhyncin II
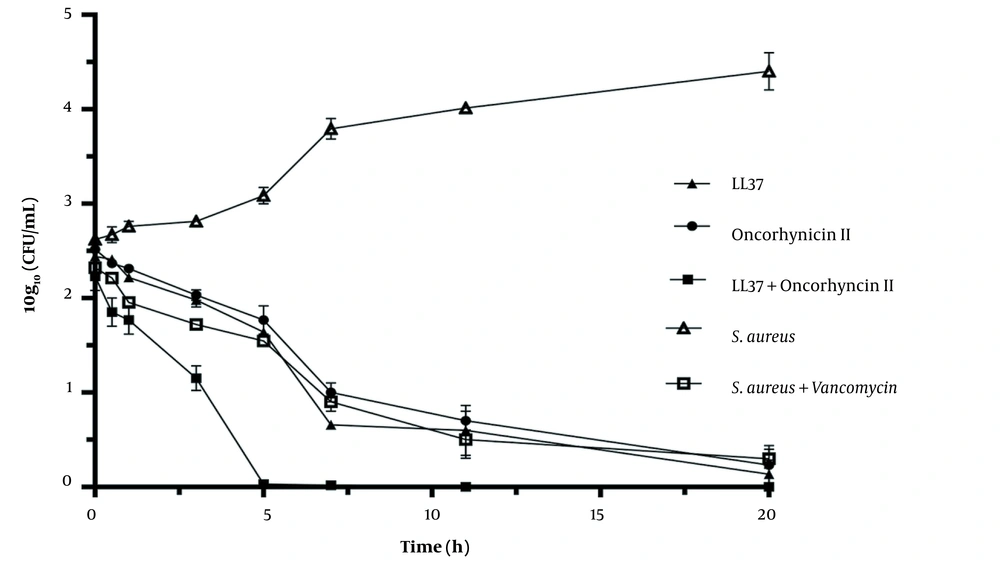
The results of the time-kill assays for S. aureus at 2 × MIC for LL-37 and oncorhyncin II are presented in terms of the changes in the log 10 CFU/mL ofviable cells in Figure 2. The number of viable cells was reduced pointy with more than 3 reductions in log 10 CFU/mL by LL-37 and oncorhyncin II alone at 2 × MIC within 7 hours, and the greatest cell reduction occurred in 20 hours against S. aureus. The treatment of S. aureus at 2 × MIC of vancomycin was reduced by more than 3 log 10 CFU/mL in 11 hours. While increasing the treatment time up to 5 hours for a combination of LL-37 and oncorhyncin II showed a considerable reduction in viable cells and the existence of a time, the trend was proved for these antimicrobial agents. Statistical analysis and the mean comparison confirmed a very significant difference among defined groups (P < 0.05).
Bacterial-killing kinetics for Staphylococcus aureus at 2 × MIC of LL-37 and oncorhyncin II. Open triangles represent the control; open squares represent S. aureus + vancomycin (1.42 μg/mL); filled triangles represent LL-37 (61.2 μg/mL); filled circles represent oncorhyncin II (95.86 μg/mL), and filled squares represent the combination of LL-37 (7.64 μg/mL) and oncorhyncin II (47.92 μg/mL).
4.4. Growth Kinetic Curves of LL-37 and Oncorhyncin II
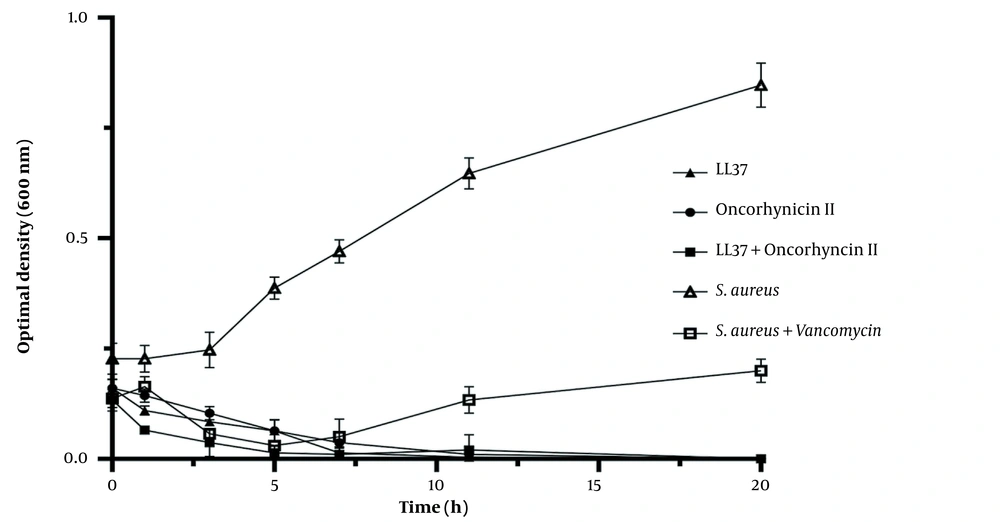
The turbidity of bacterial cultures exposed to 2 × MIC of LL-37 and oncorhyncin II was measured throughout time using a spectrophotometer to determine the mechanism of action of LL-37 and oncorhyncin II against S. aureus. After 7 hours, LL-37 decreased S. aureus suspensions’ turbidity by more than 95%. Seven hours after treatment with oncorhyncin II, there was a 50% reduction in cell turbidity. When LL-37 was coupled with oncorhyncin II, the turbidity of S. aureus suspensions decreased by more than 50% in the first hour and 100% after 3 hours (Figure 3). According to Figure 3, after 5 hours of treatment of S. aureus at 2 × MIC of vancomycin, the turbidity of the bacterial suspension increased, presumably due to the reduction of vancomycin efficacy. There were statistically significant differences between each group (P < 0.05).
Bacterial growth kinetics for Staphylococcus aureus at 2 × MIC of LL-37 and oncorhyncin II. Open triangles represent control; open squares represent S. aureus + vancomycin (1.42 μg/mL); filled triangles represent LL-37 (61.2 μg/mL); filled circles represent oncorhyncin II (95.86 μg/mL), and filled squares represent the combination of LL-37 (7.64 μg/mL) and oncorhyncin II (47.92 μg/mL).
5. Discussion
Antimicrobial peptides possess antibacterial activity against a variety of bacterial strains and can offset the inefficacy of conventional treatments in the face of antibiotic resistance (35). LL-37 and oncorhyncin II are strong AMPs with diverse antimicrobial activities (23, 24). In the present investigation, E. coli DE3 subspecies BL21 was able to synthesize recombinant LL-37 and oncorhyncin II AMPs. Recombinant proteins synthesized in this strain are not a breakdown because this strain lacks membrane-bound proteases. Following the purification steps, the recombinant protein that was over 70% pure was obtained through nickel affinity chromatography (36). There are drawbacks to employing recombinant technology to produce these proteins, such as a large reduction in their activities due to the loss of their natural structures during the manufacturing and purification processes. To resolve this issue, the protein structure must be refolded after the purification step by utilizing the dialysis process and eliminating factors such as urea. This process enhances the protein’s function.
In the present study, experiments on antimicrobial activity of recombinants of LL-37 and oncorhyncin II demonstrated that refolding in exchange buffers with pH = 7 for LL-37 and with pH = 8.5 for oncorhyncin II, as well as the addition of 0.1 M arginine and 0.1 M proline amino acids, resulted in the production of more active and positive proteins, which has been validated in previous studies (18, 25). By interacting with the hydrophobic surface of proteins, proline enhances their stability, prevents their association, and accelerates their refolding. Arginine suppresses the formation of inclusion bodies by interacting with the hydrophilic surface of the peptide, thus preventing its accumulation.
The important point is that there is a clear correlation between the isoelectric point of AMPs and their capacity to refold. Consequently, the presence of arginine and proline amino acids allows the protein to exactly refold at its optimum pH (37). The recombinant of LL-37 and oncorhyncin II, which both possess a net positive charge, is better able to bind through strong electrostatic attraction to the negatively charged bacterial cell walls (38). In this study, the final produced refolded LL-37 and oncorhyncin II recombinants were effective against gram-positive bacteria S. aureus; as predicted, the combination of LL-37 and oncorhyncin II had a lower MIC than LL-37 or oncorhyncin II alone due to significant synergistic antibacterial activity, which was corroborated by the checkerboard assay.
Numerous studies have demonstrated that LL-37 alone is insufficient to achieve the desired level of bacterial inhibition. However, when combined with exogenous antibiotics from the bactericidal family, especially those that alter the bacterial wall structure, LL-37 produces a significant reduction in the MIC values and synergy or additive effects against gram-positive bacteria, which can be used as a therapeutic advantage to raise antibiotics effectiveness and decrease their toxicities by lowering the dose required (14, 39-41). Shurko et al. observed that the MIC of LL-37 against S. aureus (ATCC 25923) was 512 µg/mL. In this investigation, LL-37 and its short-chain derivatives (LL-13 and LL-17) showed great synergy with vancomycin against vancomycin-resistant S. aureus (VRSA). LL-13 and LL-17 lowered the MIC of vancomycin substantially (42).
Any in vitro synergistic effects must be evaluated in vivo to confirm that the same interaction holds true in animal infection models. However, several investigations in the most up-to-date scientific literature have confirmed that LL-37 is somewhat more efficient against gram-negative bacteria than gram-positive and that its efficacy is generally greater, especially under more robust circumstances (high salt or complete medium) (43-45). For example, in a study (39), the MIC and MBC values of LL-37 against S. aureus (meticillin-sensitive S. aureus; MSSA and methicillin-resistant S. aureus; MRSA) and Pseudomonas aeruginosa (antibiotic-sensitive P. aeruginosa; ASPA and multidrug-resistant P. aeruginosa; MDRPA) were > 128 μg/mL and 32 - 64 µg/mL, respectively. Also, this study confirmed that the toxicity of LL-37 to eukaryotic cells was at a concentration of > 65 μg/mL. Therefore, according to the MIC values obtained from the present study, which are lower than the MIC values in previous studies, there are no cytotoxic effects at this concentration. In addition, LL-37’s anti-biofilm activity is higher than its killing potency, making it suitable for treating chronic infectious illnesses (42, 44, 46). Here, we confirmed the partial synergistic effects of the two investigated AMPs against S. aureus using checkerboard, time-kill, and growth kinetic experiments.
Kinetic and time-kill studies performed by Noore et al. showed that LL-37 killed considerably more S. aureus in the stationary phase than in the log phase; thus, LL-37 eliminated all S. aureus bacteria during the first hour of exposure (47). In addition, in a kinetics analysis conducted by Kang et al., it was demonstrated that increasing the treatment period for LL-37 up to 60 minutes led to a significant increase in log reductions in CFU (48). These results are in line with the findings of this inquiry. It appears that the oncorhyncin II peptide, like other recombinant AMPs, possesses potent antimicrobial properties for the eradication of S. aureus, as demonstrated by the results of the current study and earlier research by Jafari et al. (25). Thus, based on the time-kill assay results, the antibacterial effects of this AMP were similar to the vancomycin against the desired bacterium, though treatment with the combination of these AMPs caused a sharp decline in the number of bacteria in a shorter time.
The results obtained from other previous studies have confirmed that histone-derived proteins have extensive antimicrobial activity on gram-positive and gram-negative bacteria (49). Fernandes et al. demonstrated that histone-derived oncorhyncin II, obtained from the skin secretion of rainbow trout (O. mykiss), had considerable antibacterial activity against gram-positive and gram-negative bacteria (23). Altogether, in the antibacterial assays, a combination of the recombinant peptides LL-37 and oncorhyncin II, compared with vancomycin as a control antibiotic, represented more notable bactericidal potential in a shorter time.
5.1. Conclusions
The synergistic effects of LL-37 and oncorhyncin II AMPs against S. aureus (ATCC25923) were examined for the first time. The decreased MIC, checkerboard, time-kill, and growth kinetic assays showed that these peptides had potentially strong and rapid antibacterial activity against the target bacterium. The results demonstrated that AMPs could be used as novel antibiotics, either alone or in combination with each other or in combination with previous antibiotics, to treat infections caused by S. aureus. It also seems that the results of the effectiveness of these peptides need clinical trials.