1. Background
Neonatal sepsis is a leading cause of neonatal morbidity and mortality, accounting for approximately 22% of all neonatal deaths worldwide (1). Based on population-level studies conducted over the last two decades, the global estimate of neonatal sepsis incidence was 2202 per 100,000 live births (2). It has been discovered that the bacteria that are causing sepsis change over time and depend on geographical conditions. In developed countries, as opposed to developing countries, Gram-positive bacteria are the leading cause of neonatal sepsis (3, 4). According to Akya et al., most bacterial agents (66%) causing sepsis in Iran are Gram-negative bacteria. Klebsiella pneumonia and Pseudomonas aeruginosa are the most common (5). On the other hand, Acinetobacter baumannii, K. pneumonia, P. aeruginosa, and Escherichia coli are the most common causes of neonatal sepsis with Gram-negative bacteria. These bacteria have high rates of multidrug resistance in South Asia (6, 7).
Infection with carbapenem-resistant Gram-negative bacteria is increasing globally, posing a significant challenge to healthcare systems (6-8). Neonates admitted to neonatal intensive care units (NICU) are susceptible to colonization and infection with various pathogens, including multidrug-resistant Gram-negative bacteria, resulting in considerable morbidity and mortality rates in ICUs (8-12). Treating neonatal sepsis is challenging for clinicians because it should be quick, effective against the pathogen, and safe for the infant. This challenge is becoming more difficult as the number of multidrug-resistant organisms (MDROs) increases (13).
The empirical regimen for neonatal sepsis treatment includes a combination of ampicillin and gentamicin (or amikacin), a variety of carbapenems, and vancomycin for severe cases of multidrug-resistant organisms. However, the emergence of carbapenem-resistant bacteria poses a threat to this treatment regimen. Because of their global prevalence, carbapenemase-producing bacteria are particularly concerned (14).
2. Objectives
According to the available data, no studies on neonates with carbapenem-resistant Gram-negative bacterial sepsis have been conducted in Iran. Given the importance of determining the prevalence and outcome of this type of sepsis, which has a high mortality rate due to a lack of appropriate and available antibiotics, we decided to conduct a study in the NICU of Imam Khomeini Hospital in Ahvaz, the capital city of Khuzestan province in southwest Iran.
3. Methods
This retrospective cross-sectional descriptive study investigated the frequency, bacteriologic profile, and outcome of neonatal sepsis with carbapenem-resistant Gram-negative organisms in patients referred to the NICU of Imam Khomeini Hospital in Ahvaz, the largest referral training center in Khoozestan province, from April 2018 to June 2019. The NICU in the hospital is a tertiary care unit with inborn neonates accounting for more than 95% of all admitted cases. The ward has 45 beds and admits between 2000 and 2500 neonates annually. Demographic information for mothers and neonates includes gestational age, mother’s age, pregnancy rank, number of fetuses, delivery method, sex, and birth weight. Clinical and lab data include a five-minute Apgar score, intubation in the delivery room and the NICU, mechanical ventilation in the first three days of life, status at discharge, type of isolated bacteria, and the antibiogram report, particularly for carbapenem.
The data was extracted from the patient records, and then the demographic, clinical, and bacterial information was compared between carbapenem-resistant and carbapenem-sensitive groups. This center does not routinely screen for group B Streptococcus. Pregnant women admitted to the obstetric ward with symptoms of premature rupture of membranes (PROM) are given intravenous clindamycin. All pregnant women with a gestational age of 34 weeks or less at risk of early delivery are routinely given two doses of IV betamethasone separated by 24 hours. The current study includes all neonates born in this hospital with clinical symptoms of sepsis and positive blood cultures caused by Gram-negative bacteria. Exclusion criteria include neonates born in other hospitals having significant congenital anomalies, chromosomal abnormalities, metabolic errors, and incomplete information.
Based on the gestational age, the neonates were divided into two groups: Preterm (less than 37 weeks) and term (equal to or greater than 37 weeks). They were also divided into four groups based on birth weight:
- Normal weight (2500 g to 4000 g)
- Low birth weight (1500 g to 2499 g)
- Very low birth weight (1000 g to 1499 g)
- Extremely low birth weight (less than 1000 g).
Furthermore, the neonates were divided into two groups based on the onset time of the sepsis:
- Neonates with early-onset sepsis (within 72 ≤ hours of birth)
- Neonates with late-onset sepsis (> 72 hours of birth).
- Blood cultures are performed on all infants admitted to the study ward with clinical signs of sepsis (including lethargy, hypotonia, respiratory distress, inability to feed, and body temperature instability), feeling unwell during hospitalization, and showing clinical signs of sepsis or maternal risk factors such as PROM for more than 24 hours, or symptoms of chorioamnionitis (15).
A trained nurse performed this procedure following the department’s study protocol under aseptic conditions. After washing her hands, wearing sterile gloves and a mask, the sampling site is first disinfected with a 10% betadine solution. The area is disinfected with 70% ethyl alcohol after two minutes and sterilized after one minute. At the sampling site, a peripheral vein of the upper or lower limb is chosen, and at least 1 mL of blood is taken and injected into a vial of blood culture medium (Mirmedia, Iran) and gently shaken before being stored under sterile conditions. It is then transferred and kept in an incubator at 35 - 37 degrees in the hospital laboratory. Bacterial growth was evaluated every day in the sent samples. In the case of growing organism signs, they were transferred from the liquid medium to the solid medium.
The organism subculture and detection method were based on the description of Winn et al. (16). The automated system is not used to evaluate blood cultures on this ward. The Kirby-Bauer-Disk diffusion method is used to determine antibiotic sensitivity based on Clinical and Laboratory Standards Institute (CLSI) guidelines (17). A combination of ampicillin and aminoglycoside (gentamicin or amikacin) is used for early-onset neonatal sepsis. In contrast, vancomycin and cefotaxime are used in late-onset sepsis (nosocomial sepsis), followed by carbapenems and vancomycin in antibiotic-resistant cases. The Centers for Disease Control and Prevention (CDC) defined carbapenem-resistant Enterobacteriaceae with resistance to any carbapenem as resistance to meropenem and imipenem in the unit under study (18). Under the guidelines of the CLSI for a minimum inhibitory dose (MIC), resistance to carbapenems during the study was considered > 4 μg/mL for imipenem and meropenem. Furthermore, carbapenem sensitivity was set at < 1 μg/mL for imipenem and meropenem (17).
The antibiotic protocol of colistin and meropenem is used to treat the affected patient, according to a microbiology report indicating the growth of an organism resistant to carbapenems.
3.1. Statistical Analysis
Descriptive statistics were used to calculate carbapenem resistance bacteria (CRB) infection rates and all-cause mortality in infants with CRB. The prevalence rate was calculated per 1000 patients, and the mortality rate per 100 patients with the disease. Logistic regression analysis compared mortality rates between neonatal factors and CRB and carbapenem-sensitive bacteria (CSB) Gram-negative infections. When the p-value was less than 0.05, differences between the two groups were considered statistically significant. IBM SPSS version 22 was used for statistical analysis.
4. Results
During the study, 2704 patients were admitted to the NICU, with 113 cases having positive blood cultures of Gram-negative bacteria. Positive Gram-negative bacteria blood cultures and carbapenem-resistant cases per 1000 admitted cases in the NICU were 23.8 (2.38%) and 15.2 (1.52%), respectively. 88.5% of the 113 neonates with positive blood cultures were preterm. Male neonates made up 58.4% of the total, while female neonates made up 47%, with a male-to-female neonate ratio of 1.4:1. The youngest and oldest mothers were 16 and 48 years old, respectively, with a median age of 28.8. The lowest birth weight of the neonates was 600 grams, and the highest birth weight was 3650 grams, with a median weight of 1550.
The neonates with very low birth weights accounted for 62% of the neonates studied. The median gestational age was 32 weeks, with a minimum of 24 weeks and a maximum of 41 weeks. The cesarean section to vaginal delivery ratio was 3.1:1 in the samples (Table 1). The most common Gram-negative bacteria in the bacteriological examination were Acinetobacter spp., with 54% positive blood cultures, and the least common was Enterobacter spp., with 6% positive blood cultures.
| No. (%) | |
|---|---|
| Age of mothers (y) | |
| Less than 20 | 10 (8.8) |
| 20 to 35 | 92 (81.4) |
| More than 35 | 11 (9.8) |
| Pregnancy grade | |
| 1 | 42 (37) |
| 2 to 4 | 51 (45) |
| More than 4 | 20 (18) |
| Method of childbirth | |
| Cesarean section | 88 (78) |
| Vaginal | 25 (22) |
| Gestational age (week) | |
| Less than 28 | 16 (14) |
| 28 to 32 | 47 (42) |
| 33 to 36 | 37 (33) |
| 37 and more | 13 (11) |
| Birth weight (g) | |
| Less than 1000 | 17 (15) |
| 1000 to 1499 | 38 (33.6) |
| 1500 to 2499 | 42 (37.1) |
| 2500 and more | 16 (14.3) |
| Gender | |
| Male | 66 (58) |
| Female | 47 (42) |
| 5-minute Apgar | |
| Less than 7 | 41 (36) |
| 7 and greater | 72 (64) |
| Intubation in the delivery room | |
| No | 84 (74.4) |
| Yes | 29 (25.6) |
| Mechanical ventilation in the first 3 days of hospitalization | |
| Yes | 74 (65.4) |
| No | 39 (34.6) |
| Central venous catheter | |
| Yes | 47 (41.5) |
| No | 66 (58.5) |
| Discharge status | |
| Alive | 57 (50.4) |
| Dead | 56 (49.6) |
| Total | 113 (100) |
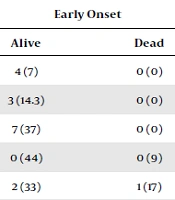
All cases of neonatal sepsis caused by Pseudomonas had a late onset. Acinetobacter (85.2%) and Pseudomonas (0%) had the highest and lowest carbapenem resistance rates. Acinetobacter isolates were resistant to ampicillin, gentamicin, amikacin, and cefotaxime. Other Gram-negative bacteria isolates were significantly resistant to the first-line treatment protocol (Table 2). Late-onset sepsis had significantly higher carbapenem resistance than early (72.7% vs. 51%, P-value = 0.022). Acinetobacter and Pseudomonas caused the highest and lowest mortality rates in Gram-negative bacterial sepsis (70.3% and 18%).
The mortality rate was significantly associated with carbapenem resistance, so 89.3% of neonates with a positive culture of carbapenem resistance died. In comparison, 10.7% of neonates with a positive culture of carbapenem sensitivity died (P-value = 0.000, AOR = 14.210, 95% Cl (4.017 - 50.255). The statistical analysis also looked at the link between mortality and neonatal factors. It showed a clear link between gestational age of fewer than 32 weeks and mortality (P-value = 0.000, 95% CI (1.985 - 9.906) AOR = 4.435). The time of sepsis (early and late) had no significant relationship with mortality (P-value = 0.497, 95% CI (0.365 - 1.630), AOR = 0.771) (Table 3).
| Bacteria | Resistance | Sensitive | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Early Onset | Late-Onset | Early Onset | Late-Onset | ||||||
| Alive | Dead | Alive | Dead | Alive | Dead | Alive | Dead | ||
| Acinetobacter spp | 1 (1.8) | 14 (27) | 7 (16) | 24 (42) | 4 (7) | 0 (0) | 4 (7) | 0 (0) | 54 (100) |
| Klebsiella pneumonaie | 4 (9) | 0 (0) | 4 (13) | 5 (35) | 3 (14.3) | 0 (0) | 7 (26) | 0 (4.3) | 23 (100) |
| Escherichia coli | 0 (0) | 3 (31.5) | 3 (0) | 3 (21) | 7 (37) | 0 (0) | 2 (5.26) | 1 (10.5) | 19 (100) |
| Pseudomonas aeruginosa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (44) | 0 (9) | 9 (36) | 2 (9) | 11 (100) |
| Enterobacter spp | 0 (0) | 1 (17) | 0 (0) | 2 (33) | 2 (33) | 1 (17) | 0 (0) | 0 (0) | 6 (100) |
| Total | 5 | 19 | 15 | 33 | 16 | 1 | 21 | 3 | 113 (100) |
a Values are expressed as No. (%).
| Variables | Outcome | Odds Ratio | CI (%95) | P-Value | |
|---|---|---|---|---|---|
| Live, 57 | Death, 56 | ||||
| Univariate Analysis | |||||
| Infants gestational age (week) | |||||
| 32 or more | 23 (40.4) | 42 (75) | 1 | ||
| Less than 32 | 34 (59.6) | 14 (25) | 4.435 | 1.985 - 9.906 | 0.000 |
| Mode of delivery | |||||
| Cesarean section | 45 (78.9) | 43 (76.7) | 1 | ||
| Vaginal | 12 (21.1) | 13 (23.31) | 1.134 | 0.466 - 2.758 | 0.782 |
| Birth weight (g) | |||||
| 1500 or more | 39 (68.4) | 19 (33.9) | 1 | ||
| Less than1500 | 18 (31.6) | 37 (66.1) | 4.219 | 1.922 - 9.260 | 0.000 |
| Sex | |||||
| Female | 26 (45.6) | 21 (37.5) | 1 | ||
| Male | 31 (54.4) | 35 (62.5) | 1.398 | 0.659 - 2.963 | 0.382 |
| 5-minute Apgar less than 7 | |||||
| No | 47 (82.5) | 25 (44.6) | 1 | ||
| Yes | 10 (17.5) | 31 (55.4) | 5.828 | 2.461 - 13.803 | 0.000 |
| Intubation in the delivery room | |||||
| No | 8 (14) | 21 (37.5) | 1 | ||
| Yes | 49 (86) | 35 (62.5) | 3.675 | 1.461 - 9.246 | 0.006 |
| Mechanical ventilation in the first 3 days of life | |||||
| No | 34 (59.6) | 5 (8.9) | 1 | ||
| Yes | 23 (40.4) | 51 (91.1) | 15.078 | 5.224 - 43.522 | 0.000 |
| Sepsis time (day) | |||||
| Early: First 3 | 26 (45.6) | 22 (39.3) | 1 | ||
| Late: After 3 | 31 (54.4) | 34 (60.7) | 0.771 | 0.365 – 1.630 | 0.497 |
| Duration of hospitalization (day) | |||||
| 7 or less | 20 (36.4) | 42 (85.7) | 1 | ||
| More than 7 | 35 (63.6) | 14 (14.3) | 10.500 | 3.978 - 27.715 | 0.000 |
| Resistance to carbapenems | |||||
| No | 35 (85.3) | 6 (14.7) | 1 | ||
| Yes | 22 (30.5) | 50 (69.5) | 50.308 | 6.698 - 377.876 | 0.000 |
| Multivariate Analysis | |||||
| Infants gestational age (week) | |||||
| 32 or more | 23 (40.4) | 42 (75) | 1 | ||
| Less than 32 | 34 (59.6) | 14 (25) | 3.517 | 1.149 - 10.763 | 0.028 |
| 5-minute Apgar less than 7 | |||||
| No | 47 (82.5) | 25 (44.6) | 1 | ||
| Yes | 10 (17.5) | 31 (55.4) | 4.086 | 1.287 - 12.970 | 0.017 |
| Mechanical ventilation in the first 3 days of life | |||||
| No | 34 (59.6) | 5 (8.9) | 1 | ||
| Yes | 23 (40.4) | 51 (91.1) | 10.512 | 2.809 - 39.332 | 0.000 |
| Resistance to carbapenems | |||||
| No | 35 (61.4) | 6 (10.7) | 1 | ||
| Yes | 22 (38.6) | 50 (89.3) | 14.210 | 4.017 - 50.266 | 0.000 |
a Values are expressed as No. (%) unless otherwise indicated.
5. Discussion
The current study was carried out to examine the link between neonatal sepsis and Gram-negative bacteria, which have a high mortality rate. According to the findings, the rate of positive blood cultures with Gram-negative bacteria and carbapenem-resistant cases per 1000 admitted cases in the NICU was 23.8 and 15.2, respectively. There were no comparable data to compare with the current study’s findings. The prevalence of sepsis in female neonates in this study was 42%, which, consistent with the findings of other studies, was lower than in male neonates. Yadav et al. reported similar rates for female neonates (47%) (19). Male neonates are more sensitive to adverse perinatal and postnatal environmental conditions and are more likely to be born preterm and with a lower birth weight, which increases the risk of neonatal sepsis (20). Additionally, the more initial respiratory support required by male neonates may lead to poorer outcomes (21).
The average gestational age and birth weight of the infants in this study were 31.7 weeks and 1689 grams, respectively. The Delhi neonatal infection study (DeNIS) from India (22) and Cagan et al. from Turkey (23) have reported the average gestational age and birth weight of newborns to be 36 and 33.6 weeks, respectively, and the birth weight to be 2,211 and 2,452 grams. It demonstrates a significant difference. As previously stated, the newborns’ lower gestational age and birth weight in this study are because almost all high-risk pregnancies in our province are observed in this obstetrics and gynecology ward. In this study, three isolated organisms, in order of prevalence, were Acinetobacter, K. pneumonia, and Escherichia coli. The frequency of Gram-negative bacteria causing sepsis in our study is consistent with the DeNIS Cohort study (22). The similarity of sepsis-causing organisms in both studies could be attributed to the same geographical, health, and genetic conditions and human and non-human facilities.
In the current study, the maximum positive blood cultures were associated with Acinetobacter spp (47.7%), which differed significantly from the prevalence reported by Nazir (13.7%) (24). However, it was consistent with the findings of Yusef et al. (42.8%) (13). The high prevalence of Gram-negative sepsis with Acinetobacter could be attributed to factors such as the high frequency of very low birth weight, the need for non-invasive or invasive ventilation in the first 3 to 5 days of life, frequent changes to antibiotics, and the use of carbapenems, a lack of access to adequate care facilities, high bed occupancy, a shortage of trained nurses, insufficient hand washing, and the reuse of disposable respiratory circuits. In a systematic review, Okomo et al. discussed the etiological factors and causes of antibacterial resistance in neonatal sepsis. He believes geographical differences in microbiology are likely related to a diverse prevalence of maternal risk factors (including human immunodeficiency virus disease), neonatal risk factors such as prematurity, different obstetric and neonatal health care practices, and regional variation in community flora (25).
The present study found carbapenem resistance in 63.7% of sepsis patients. The values reported in the current study, Sands et al.’ analysis, and Nordberg et al.’s study in Sweden differ significantly. Sands et al. investigated neonates’ blood cultures from seven Asian and African countries and reported resistance to meropenem (13%) and imipenem (15%) in Gram-negative bacteria. According to Nordberg et al., the carbapenem resistance rate of Gram-negative bacteria causing sepsis is 0% (7, 26). In our study, Acinetobacter sepsis caused the highest carbapenem resistance (85.2%), consistent with Nazir’s finding, in which the resistance to Acinetobacter was reported as 93% (24). According to his report, carbapenems are used as the first-line treatment for many neonates in India. Our findings showed Pseudomonas had the highest carbapenem sensitivity (100%). Similarly, Pandit and Vyas conducted a study in Nepal and found 94% sensitivity to Pseudomonas (27). However, it contradicts the study by Rao et al. on 8 NICUs in China, which reported a carbapenem sensitivity of 60% (28).
This study’s overall mortality rate in infants with Gram-negative sepsis was 49.5%. It differed from Wen et al.’s systematic review and meta-analysis, which reported a mortality rate of 11% to 19% (8). However, it is consistent with Peters et al.’ study, which found a 44.3% mortality rate in infants with Gram-negative sepsis resistant to several drugs (29). In our study, the mortality rate of premature infants with a gestational age of fewer than 32 weeks was higher than that of infants with a gestational age of more than 32 weeks. This finding is comparable to studies conducted in Ethiopia and Iraq and a systematic review conducted in developing countries (30-32). Preterm neonates are more likely to die than term neonates due to a lack of various immune system components, despite many aggressive measures to preserve their lives.
In this study, the mortality rate in infants with Gram-negative sepsis resistant to carbapenems was higher than in the sensitive group. Peters et al. (29), in their cohort study on the resistance to multiple antibiotics as a risk factor for death, has considered resistance to numerous antibiotics as one of the factors increasing the probability of death in infants. Unlike our study, no significant relationship was found between mortality and antibiotic resistance in the DeNIS study (22).
The highest mortality rate in neonates and infants with sepsis was reported in Acinetobacter carbapenem-resistant sepsis (70.3%). Similarly, Sultan discovered that 76.9% of neonates with sepsis caused by carbapenem-resistant Acinetobacter died (33). However, the current findings contradict the findings of the DeNIS study, which found that patients with sepsis caused by Pseudomonas species have the highest mortality rate (78%) (22). Because of the significant frequency between Acinetobacter and other isolated organisms and the strong resistance of isolated Acinetobacter to several types of antibiotics (particularly carbapenems), a higher frequency of mortality due to sepsis with Acinetobacter can be expected.
This study was single-center research conducted in Iran's southwest. The findings cannot be generalized to other centers or regions of the country. Further, since the present study is retrospective, the information was incomplete, and some critical variables were overlooked. Therefore, prospective multicenter research with more cases is recommended to obtain more detailed results.
5.1. Conclusions
According to the findings of this study, the prevalence of carbapenem-resistant Gram-negative sepsis in this facility was high. Most infants in the study were premature, had low birth weight, were male, and required mechanical ventilation for the first three days of life. Resistance to carbapenems was significantly higher in late-onset sepsis than in early-onset sepsis. The most common cause of Gram-negative carbapenem-resistant neonatal sepsis was Acinetobacter spp. The most common cause of death was Acinetobacter bacteria. Early preterm birth, very low birth weight, Apgar scores less than seven at the fifth minute, mechanical ventilation in the first three days of life, and sepsis due to Acinetobacter were all associated with higher mortality risk.