1. Background
Hepatitis E virus (HEV) belong to the OrthohepevirusA species, Orthohepevirus genus, and Hepeviridae family (1). HEV is transmitted through the path of the fecal-oral and accounts for critical hepatitis but self-limited infection (2). Clinical manifestations of HEV infection vary from moderate to severe hepatitis among all group ages (3). High mortality rate of 25% to 31% have been reported among pregnant women (4) and 30% to 70% in patients who have chronic liver diseases (5, 6). In 2016, the world health organization (WHO) reported that there are approximately 20 million individuals who are infected with HEV, approximately 3.3 million severe cases, and 56600 mortalities annually, which occurs around the world (7).
Hepatitis E virus has been classified into 5 genotypes, although all the genotypes share 1 serotype. Genotypes 1 and 2 are considered to only infect humans, while genotypes 3 and 4 are zoonotic; in addition, genotype 5 is restricted to avian infection (8). According to the recent data, several Asian and African countries, including Iran, are classified in the high HEV endemicity (9). To date, there is no exclusive treatment for severe hepatitis E and no approved licensed vaccine exists against the HEV infection (10). Thus, based on the aforementioned data, an efficient vaccine against HEV infection should be developed for prevention of HEV infection. For the time being, no tissue culture system is available for cultivation of HEV. Therefore, molecular approaches are only alternative tools for the vaccine preparation (11).
Hepatitis E virus genome comprises 3 overlapping open reading frames (ORF) (12). ORF1 gene is responsible for enzymatic function and encodes the nonstructural proteins. ORF2 gene involves the synthesis of a viral capsid protein (72 kDa) and ORF3 gene encodes a small protein (13.5 kDa), which may have a regulatory function (13). The capsid protein, with 660 amino acids (aa), is highly immunodominant and candidates for the HEV vaccine development (14). However, it was found that the full-length capsid protein expression is insoluble and less immunological reactivity. The construction of a truncated ORF2 protein (aa 112 to 660), which can be self-assembled into virus-like particles (VLPs), induces systemic and mucosal immune responses in experimental animals (15). Thus far, different truncated forms of the ORF2 protein (aa 112 - 660, aa 112 - 607, aa 458 - 607, as well as aa 368 - 606) have been constructed and used for HEV vaccines (16-18).
Since the recombinant vaccines, including DNA vaccines, have been developed, in order to improve the vaccine potency, several factors such as codon optimization of the gene, co-administration of different adjuvants, and use of plasmids encoding a secretory protein type have been studied. The occurrence of the codon usage is diverse amongst the numerous organisms. Thus, codon optimization can steer towards an extreme expression of the target gene mimic to the host cell genes (19).
Antigen processing and presentation in cells is an important key for the activation of both humoral and cell mediate immunity (20). Since DNA vaccines can be constructed for specific cell compartments in antigenic processing, vaccination with recombinant DNA can usually activate both arms of the immune system. On the other hand, the existence of a secretary target protein in the cloned plasmids constructed by fusing target gene to the signal sequence of human tissue plasminogen activator (tPA) are found highly immunogenic for both B and T cells, than the plasmids without a secreted form (21). PADRE peptide, which is also known as a universal Pan HLA-DR epitope, can bind to various high-affinity class-2 DR molecules in major histocompatibility complexes (MHCs) and can be applicable for humans and mice (22). Based on the aforementioned data, in this study, a DNA plasmid containing truncated ORF2 (aa 112 - 607) was constructed with tPA-PADRE genes cassette. The novel chimerical pVAX1-tPA-PADRE-truncated ORF2 plasmid comprises the secretary signal sequence, which derives from tPA, and PADRE sequence, were fused at the N-terminal of truncated HEV ORF2 gene and can be used as a candidate HEV vaccine.
2. Objectives
The aim of this study was to construct the recombinant plasmid pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) as a DNA vaccine candidate and to evaluate the expression of cloned, truncated ORF2 of HEV in the pVAX1 vector transfected in HEK293 cells and Chinese hamster ovary (CHO) eukaryotic cells.
3. Methods
3.1. Ethics Statement
This study was approved with registration number 145 by the ethics committee at Ahvaz Jundishapur University of Medical Sciences.
3.2. Construction of ORF2 (aa 112 - 607) from Designed, Optimized pVAX1-tPA-PADRE-Truncated ORF2 (aa 112 - 660) Cassette of Hepatitis E Virus
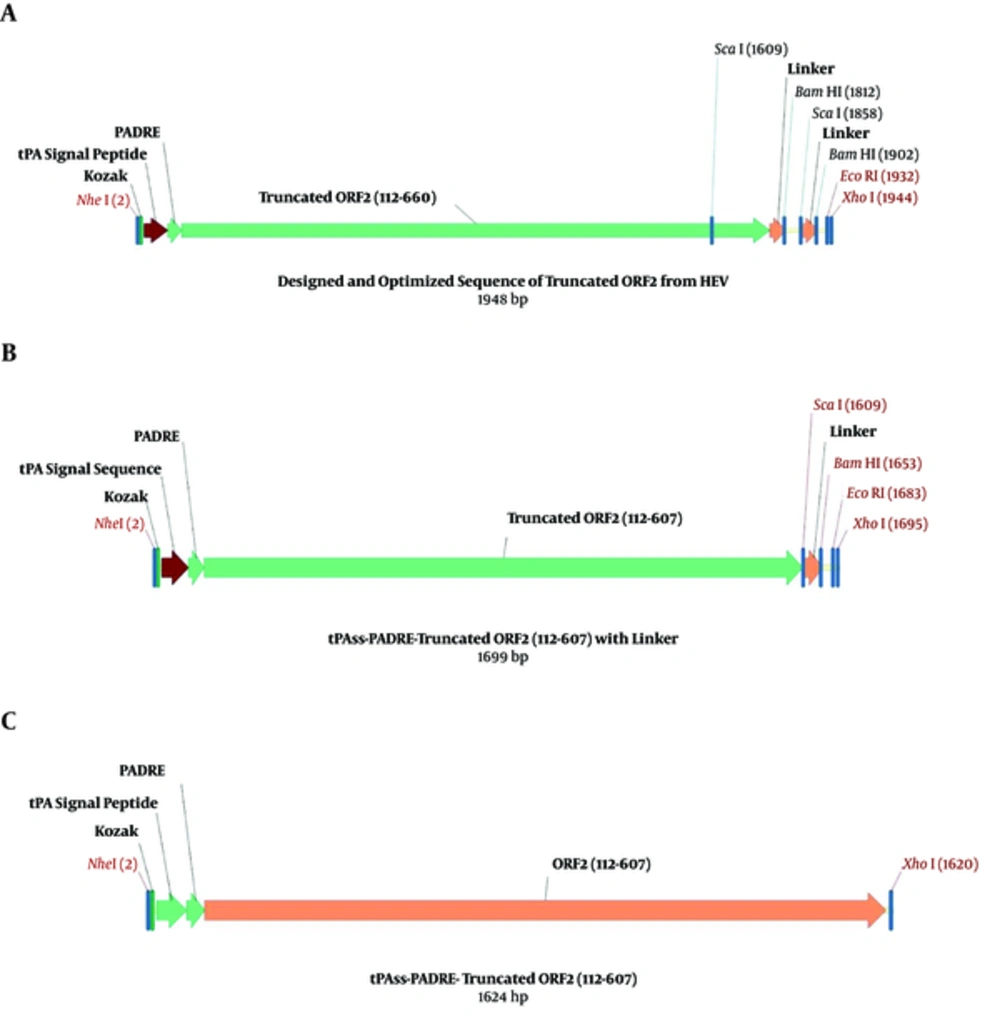
The ORF2 gene amino acid sequence (112 - 660) in HEV genotype 1 from a Pakistani strain (Sar-55) was developed from the UniProt records (accession number, P33426), designed from the Vector NTI software (version 11.5, Invitrogen, USA), optimized using GenScript software, and inserted into the pVAX1 plasmid (Invitrogen, USA), as mentioned in the previous study (23). To retrieve tPA-PADRE-truncated ORF2 (aa 112 - 607) gene fragment, 2 restriction enzyme digestion sites for ScaI were located at a position of 1609 and 1858 at the end of the cassette gene. The arrangement of fragment junctions is shown in Figure 1.
3.3. Sub-Cloning of the Codon-Optimized tPA-PADRE-Truncated ORF2 Gene Cassette in pVAX1 Eukaryotic Expression Vector
Escherichia coli strain DH5α (Biomedal Life Science, Spain) containing the pVAX1 eukaryotic expression vector with tPA-PADRE-truncated ORF2 (aa 112 - 660)-linker in the previous study (23) was cultured overnight in Luria-Bertani (LB) broth medium (Merck, Germany), consisting of 50 µg/mL of kanamycin (Sigma-Aldrich, Germany) at a temperature of 37°C. According to the kit’s manufacturer’s instructions, the pVAX1 plasmid was extracted using the QIAGEN Plasmid Mini Kit (Qiagen, Germany). The purified plasmid was digested using the ScaI restriction enzyme (New England Biolabs, USA) at 37°C for 14 hours. The QIAquick Gel Extraction Kit (Qiagen, Germany) was used to separate the linearized, digested pVAX1 plasmid, as well as the tPA-PADRE-truncated ORF2 (aa 112 - 607) gene from the agarose gel (1%); afterwards, ligation was performed by T4 DNA ligase (New England Biolabs, USA) at 15°C overnight. The recombinant plasmid pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607)-linker was assembled (Figure 1B) and transformed into competent cells of E. coli DH5α strain with 100 mM of CaCl2 (Merck, Germany) and then chosen on LB agar medium, consisting of kanamycin (50 µg/mL). Various colonies were analyzed by the colony PCR and the recombinant plasmids with the accurate restriction patterns were chosen by restriction digestion with NheI and XhoI enzymes (New England Biolabs, USA) and then used for DNA sequencing with T7 Forward and bovine growth hormone (BGH) reverse primers (by Bioneer, Korea).
The gene fragment encoding tPA-PADRE-truncated ORF2 (aa 112 - 607), with liker sequence, was PCR amplified by using the Q5 High-Fidelity DNA Polymerase (Qiagen, Germany). The order of forward primer, which included a Kozak consensus sequence, was 5’-CCAAGCTGGCTAGCTGGAGCCGCCACCATGGATGCA-3’ and the order of the reverse primer was 5’-CCCGCTCGAGTTATCACAGTACTGAGTGTGGTGCGAGGAC-3’, then cloned right into the pVAX1 expression vector using NheI and XhoI restriction sites. The recombinant plasmid was electroporated into E. coli DH5α. The cloning of the tPA-PADRE-truncated ORF2 (aa 112 - 607) gene into the pVAX1 expression vector was confirmed by enzyme digestions, colony PCR by universal primers (T7, BGH), as well as DNA sequencing. The verified construct was called pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) (Figure 1C).
3.4. Expression of the Recombinant pVAX1-tPA-PADRE-Truncated ORF2 (aa 112 - 607) in Mammalian Cells
To express the recombinant protein in the eukaryotic cells, CHO and HEK293 cells (National Cell Bank of Iran at Pasteur Institute) were transfected with the recombinant plasmid pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) by PolyFect Transfection Reagent (Qiagen, Germany) conferring to the manufacturer’s guidelines in 6 well culture plates (Nunc, Denmark). The cells were cultured in Opti-MEM medium (Gibco-Fisher Scientific, USA) without serum and antibiotics at 37°C and 5% CO2 to allow gene expression. To verify mRNA expression of truncated ORF2 gene (aa 112 - 607), RT-PCR was carried out after 48 hours transfection. In short, the over-all cellular RNA was extracted via the RNeasy Mini Kit (Qiagen, Germany). In addition, RT-PCR was then executed by a QIAGEN One Step RT-PCR Kit (Qiagen, Germany) conferring to the manufacturer’s protocol by specific primers (upstream primer: F29 (724) 5’- ATGAAAAGGGGTCTCTGC-3’ and downstream primer: R329 (1053) 5’-ATGCCCAGTACCGCTGGCACG-3’). Then, the RT-PCR product was electrophoresed on an agarose gel of 2%.
3.5. Confirmation of the Recombinant Protein Expression in Eukaryotic Cells
Indirect immunofluorescence assay (IFA) and western blotting evaluated the expression of the recombinant truncated ORF2 (aa 112 - 607) protein in the transfected CHO and HEK293 cells. An IFA using a Fluorescein Isothiocyanate (FITC) -labeled antibody was performed as previously described (23). The presence of the recombinant truncated ORF2 (aa 112 - 607, 56 kDa) protein, inside the eukaryotic cells, as well as in the supernatants (the secreted form), was also analyzed by western blotting, according to the method described previously (24).
4. Results
4.1. Construction of tPA-PADRE-Truncated ORF2 (aa 112 - 607) Gene Fragment
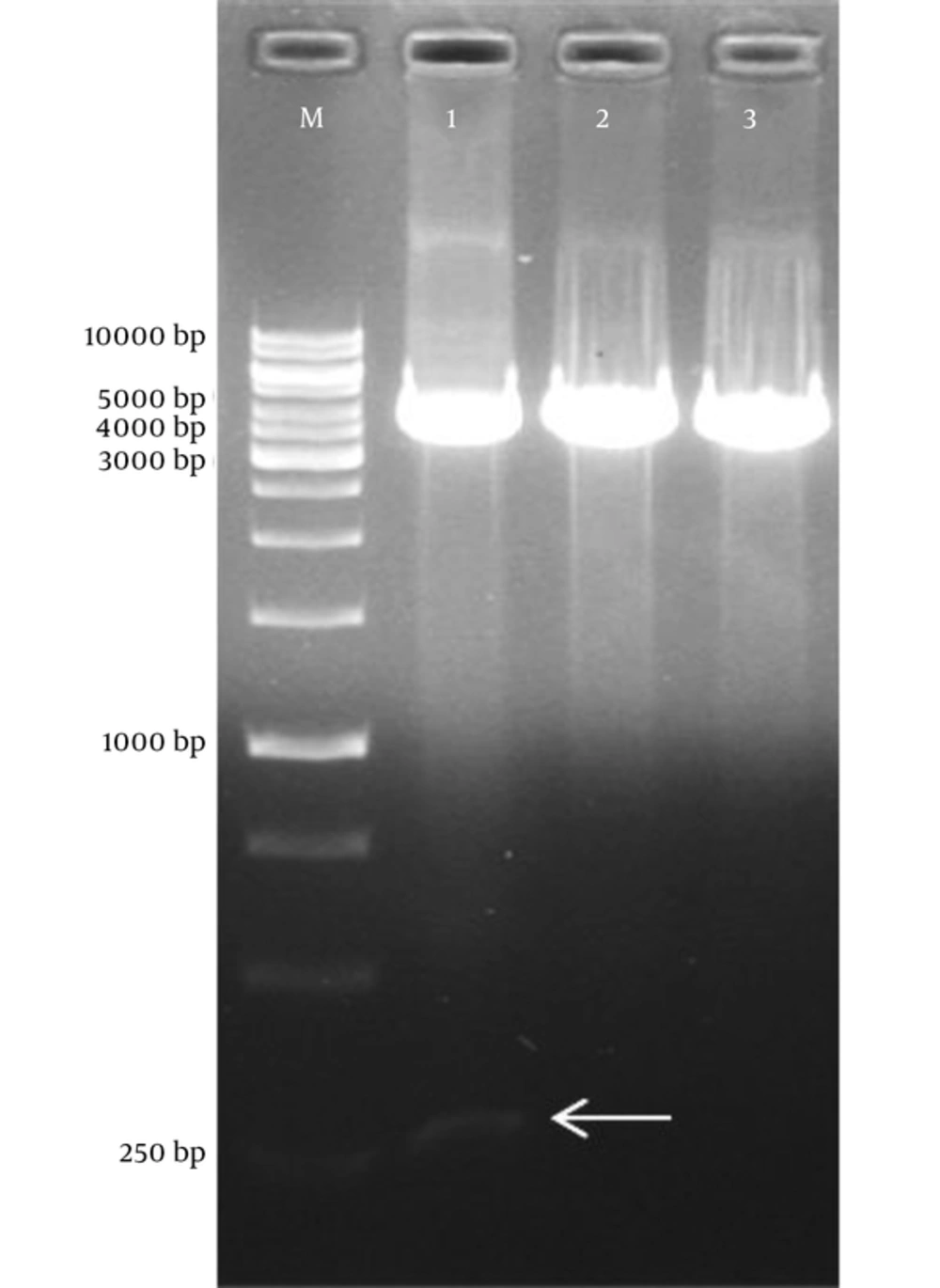
To construct tPA-PADRE-truncated ORF2 (aa 112 - 607) gene fragment with linker sequence, digestion with ScaI restriction enzyme was carried out on a pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 660) cassette that had been designed and optimized in the previous study (23). The fragment of tPA-PADRE-truncated ORF2 (aa 112 - 660) was 1948 bp in length; after digestion with ScaI, its length reduced to 1699 bp (Figure 1A and B). Figure 2 shows agarose gel electrophoresis of pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 660) digested with ScaI restriction enzyme. To remove the linker at a downstream of the gene cassette, PCR amplification was performed on pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607)-linker cassette and PCR product with 1624 bp in length was produced. This fragment was called pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) (Figure 1C).
4.2. Subcloning Results of tPA-PADRE-Truncated ORF2 (aa 112 - 607) Gene Fragment in the pVAX1 Eukaryotic Plasmid
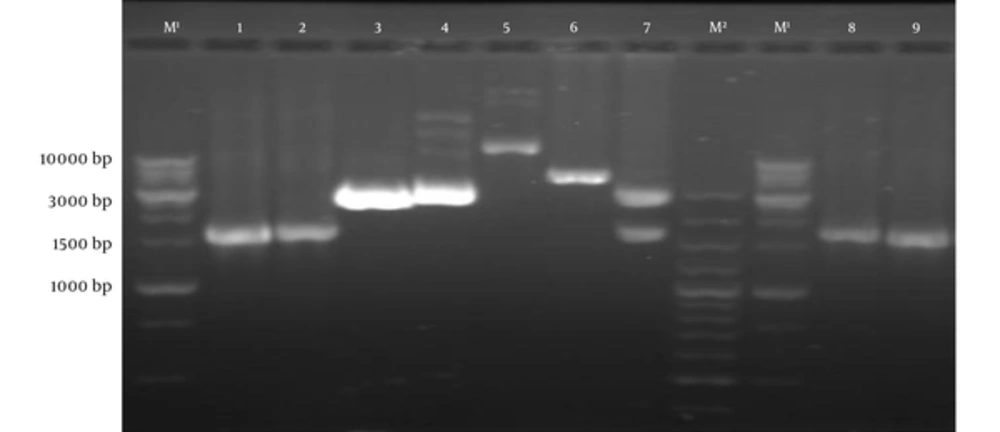
Subcloning of pVAX1 containing tPA-PADRE-truncated ORF2 (aa 112 - 607) gene fragment was approved by colony PCR and restriction enzyme digestion with NheI and XhoI enzymes (Figure 3). DNA sequencing with universal primers (T7, BGH) confirmed the cloned gene fragment.
Lane M1, DNA marker (1 kb); Lane M2, DNA marker (100 bp); Lane 1,2: PCR product of tPA-PADRE-truncated ORF2 (aa 112 - 607) by specific primers; Lane 3, pVAX1 plasmid with digestion by NheI and XhoI; Lane 4, pVAX1 plasmid with digestion by NheI; Lane 5, pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) without digestion; Lane 6, pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) digested with xhoІ; Lane 7, pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) digested with NheI and XhoI; Lane 8 and 9, PCR product of pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) plus linker by T7 and BGH primers.
4.3. Expression and Confirmation of the Recombinant tPA-PADRE-Truncated ORF2 (aa 112 - 607, 56 kDa) Protein in Mammalian Cells
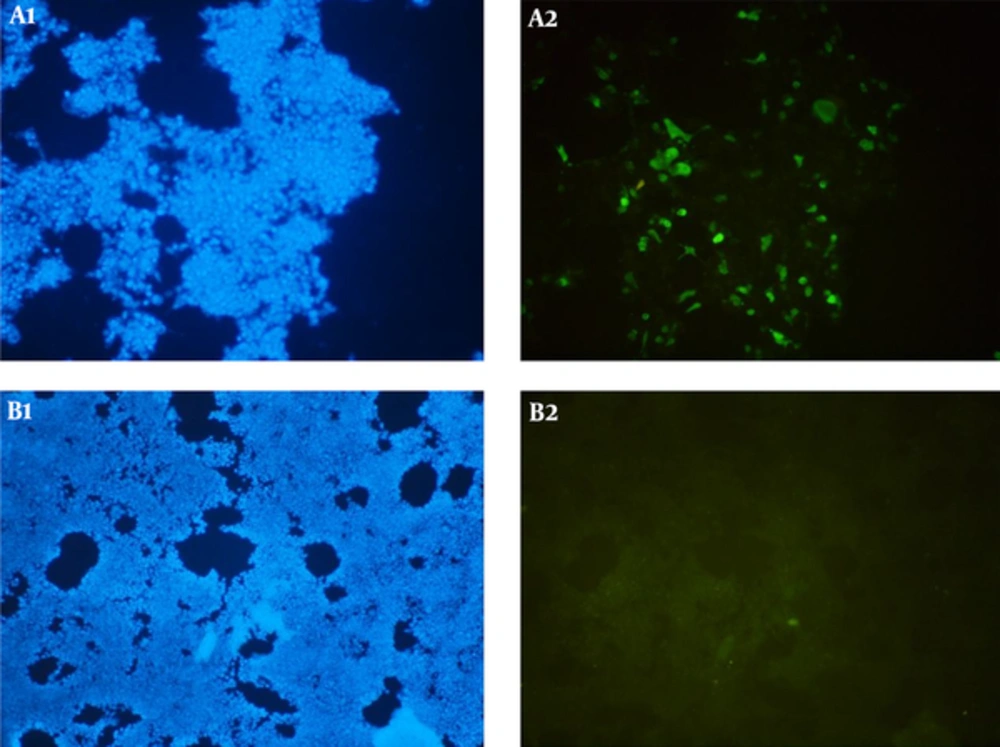
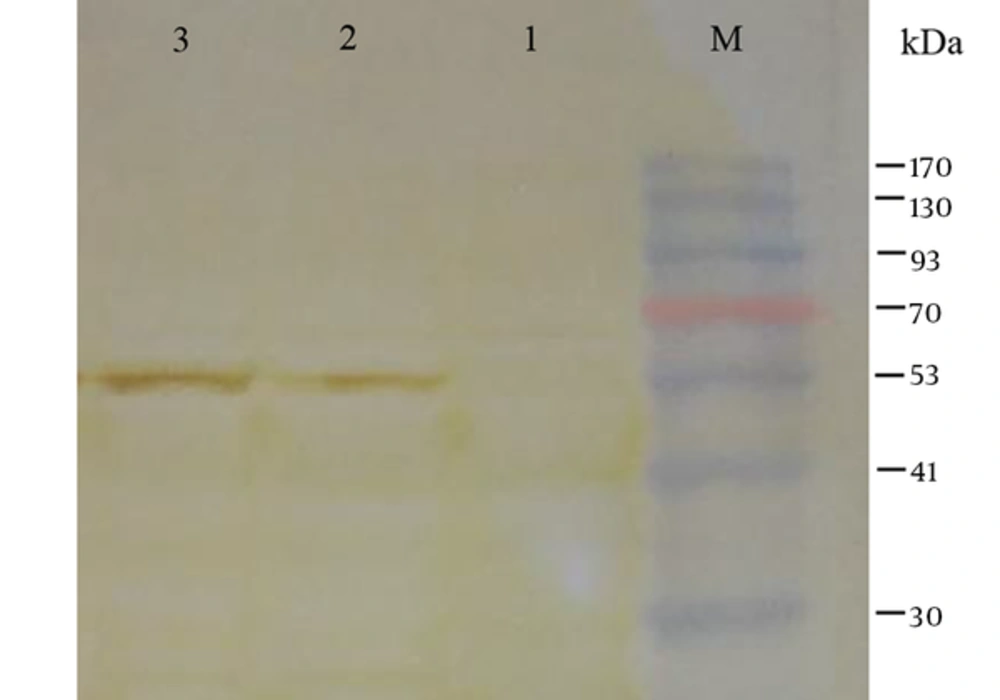
The expression of the recombinant tPA-PADRE-truncated ORF2 (aa 112 - 607, 56 kDa) protein was confirmed in the eukaryotic cells (HEK293 and CHO cells) by RT-PCR assay. The PCR product was sized to be 329 bp. The expression of tPA-PADRE-truncated ORF2 (aa 112 - 607, 56 kDa) protein in CHO cells was confirmed by IFA using anti HEV polyclonal antibody (Figure 4). Western blot analysis detected the expression of tPA-PADRE-truncated ORF2 (aa 112 - 607, 56 kDa) protein in both the supernatant and lysates collected from the transfected HEK293 cells (Figure 5).
Detection of HEV truncated ORF2 protein (aa 112 - 607, 56 kDa) in CHO cells (A1 and A2) transfected with pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) recombinant plasmid by IFA. B1 and B2, CHO cells transfected cells with pVAX1 plasmid (negative control). DAPI was used to stain the cell nuclei (A1 and B1).
Lane M, pre-stained protein ladder; Lane 1, HEK293 cells transfected with pVAX1 (as negative control); Lane 2, detection of truncated ORF2 protein (aa 112 - 607) in the supernatant of HEK293 cells transfected with pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) recombinant plasmid; Lane 3, detection of truncated ORF2 protein (aa 112 - 607) in cell lysate of HEK293 cells transfected with pVAX1-tPA-PADRE-truncated ORF2 (aa 112 - 607) recombinant plasmid. A protein band about 56 kDa corresponding to tPA-PADRE-truncated ORF2 (aa 112 - 607) recombinant protein is detected.
5. Discussion
Although most HEV infections are asymptomatic, some can cause acute hepatitis with several clinical manifestations including glomerulonephritis, thrombocytopenia, and neurological disorder (25). In addition, HEV infection can be severe in pregnant women with a high mortality and morbidity rate, especially in developing countries leading to fulminant hepatic failure, and in patients with a super-infection (1). Chronic HEV infections may also occur in immunocompromised patients (2).
Due to lack of proper treatments for HEV infections, the existence of active immunization remains only alternative for the prevention of HEV infections. Several attempts have been made to develop a HEV vaccine (26). Since there is no efficient tissue culture system for cultivation of HEV, the recombinant DNA technology is the only approach in developing an HEV vaccine (27). To achieve this, DNA vaccines have been discovered to have several advantages including cost-effective, stability, and higher immunogenicity of a target antigen (28). Numerous reports revealed that the effectiveness of immune responses have been stimulated by DNA vaccines (29). The DNA-based vaccine was found to stimulate the CD4+ T helper cell immune responses, following the activated CD4+ T helper cells outcome in the production of CD8+ T cell immune responses, as well as the memory T cell responses (30).
Some studies show that the HEV ORF2-induced antibody exists for long periods in animals as well as humans and shows a cross-reaction among different HEV genotypes. It can also neutralize HEV in vitro. Due to its effective immunogenicity, the HEV ORF2 protein has been used as an antigen for all vaccine research studies until now (10). DNA vaccine studies have revealed that the codon optimization could enhance the expression of recombinant proteins (31). The present study displays that the codon optimization for expressing the recombinant truncated ORF2 gene is effective in eukaryotic cells, which is consistent with the previous report (23).
Several studies demonstrate that the expression of different target genes can be highly efficiently achieved by the pVAX1 vector. Using pVAX1 for designing and constructing the target gene has several advantages such as stability and cost-effectiveness, especially for developing countries (32). To enhance the protein yield, adding of Kozak sequence to the upstream of the initiation codon can increase the protein expression level. As a result, in the present study, the Kozak sequence was joined with truncated ORF2 (aa 112 - 607, 56 kDa) gene sequence at N-terminal exactly before the start codon of the gene. The study conducted by Farshadpour et al. shows the proper expression of truncated ORF2 (aa 112 - 660, 63 kDa) in HEK293 and CHO cells using the Kozak sequence (23). The presence of the signal sequence of tPA in a target gene leads to construct a secretary form of the protein, which makes it better to present to both T and B cells (21). In other words, the secretion of a protein will boost the antigen presentation via MHC class II, which result in enhancing the activation of antigen-specific CD4+ T-cells. Thus, in the present study, the secretory tissue plasminogen activator (tPA) sequence was used in DNA vaccine construction. In addition, the existence of PADRE sequence improves the presentation of a target peptide via MHC class II for CTL responses. Some studies revealed that PADRE peptide is capable of attaching to the alternates of human MHC class II molecules DR and stimulate helper-mediated immune responses in human species, as well as mice. It can also enhance the potency of vaccines in preclinical models (33, 34). Therefore, in the present study, the sequence of tPA and PADRE were added respectively after the Kozak sequence to the N-terminal sequence of truncated ORF2 (aa 112 - 607) gene.
6. Conclusion
In conclusion, this study showed that the encoded truncated form of HEV capsid protein (ORF2) (aa 112 - 607, 56 kDa) was efficaciously expressed in eukaryotic cells; although, the immunogenicity of pVAX1 containing truncated ORF2 (aa 112 - 607) requires to assess in vivo immunologically and cell mediated immune response.