1. Background
Blastocystis, the most common unicellular eukaryote colonized in the large intestine of both humans and nonhuman hosts has a worldwide distribution (1-3). More than 100 years ago Blastocystis was described by Allexief, but its pathogenicity still remains controversial (4, 5). The parasite was frequently reported in both healthy individuals and symptomatic patients (6). Gastrointestinal (GI) symptoms such as abdominal pain, watery diarrhea, vomiting, and flatulence have been attributed to the Blastocystis infection (7). In developing countries, Blastocystis has a higher prevalence (53.8%) than developed countries (3.3%) (1, 8, 9). Various factors such as poor hygiene, close contact to infected animals and eating contaminated food or water may cause different prevalence (3). Blastocystis mainly infects older children and adults, particularly, people in the age range of 30 - 50 years (4, 10).
The main rout of the transmission in humans is fecal-oral, but molecular studies revealed that a zoonotic transmission can occur (11). Based on studies conducted on a genetic diversity using the small subunit rDNA (SSU-rDNA), at least 17 subtypes (STs) of Blastocystis were identified in humans and animals (2). From these, nine subtypes (ST1-ST9) were identified in humans and the other eight subtypes were found only in animals (9). The geographic distribution of the identified subtypes in human might be affected by different factors such as region, weather, contact with reservoir hosts, cultural behaviors and transmission route (1). Among identified nine subtypes in humans, the most common are STs 1 - 4 with approximately 90% of human infections (12), which their prevalence is vary from one country to another (13). Subtype 3 is the most common ST with a cosmopolitan distribution, but ST4 is restricted to European countries and being rare in North Africa, the Middle East, and South America (5). In addition to being the second frequent Blastocystis ST in the UK, ST4 is reported more prevalent in irritable bowel syndrome (IBS) patients (1).
Even though numerous studies have investigated to find a possible relation between Blastocystis STs and intestinal disorders, particularly IBS, the results are still very contradictory (12). The IBS is a functional GI disorder, with worldwide prevalence rates of 5% - 24% in developed and 35% - 43% in developing countries (10). Based on the diagnostic Rome III criteria, the IBS patients were classified into four subgroups of IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), IBS with bowel mixed pattern (IBS-M), and IBS unsubtyped (12). Although the pathophysiology of IBS still remains elusive (10), some intestinal protozoa such as Blastocystis sp., Dientamoeba fragilis, and Giardia intestinalis have been attributed to the etiology of IBS (10).
In Iran, information about Blastocystis subtypes is restricted to a few studies (14-17) and only one study has investigated the relationship between Blastocystis sp. and IBS (18). Despite the fact that IBS is highly prevalent in Iran and the prevalence of 1.1% - 25% has been reported among Iranian people (19), investigation of the Blastocystis prevalence and its genetic diversity among IBS patients has been neglected. Moreover, reliable information on the prevalence and genetic diversity of Blastocystis in IBS subjects is scarce.
2. Objectives
The current study aimed to evaluate the Blastocystis prevalence and determine its STs based on the SSU-rDNA gene in the patients with IBS and without IBS in comparison with healthy control subjects in Ahvaz, southwest Iran. The results might provide valuable information regarding the association of Blastocystis and its STs with GI disorders, particularly IBS.
3. Methods
3.1. Ethics Statement
The protocol of this study was reviewed and approved by the Ethical and Research Committee of Ahvaz Jundishapur University of Medical Sciences with the approval number 94126-2015. Written informed consent was provided by all adult participants, and for minors, was obtained from their parents.
3.2. Sample Collection
The current case-control study was designed to investigate the prevalence and subtype diversity of Blastocystis in subjects with GI symptoms (case) and healthy population (control) form southwest Iran. Two hundred eighty-two stool samples were collected from 152 patients with GI symptoms (67 males and 85 females), and 130 healthy volunteers (102 males and 28 females) during the years 2015 - 2016. The age of the participants ranged from eight to 86, and the mean age was 40 years. Independent samples t test revealed statistically significant differences (P = 0.01) for age between the case and control groups. A gastroenterologist selected the patients with GI disorders from subjects referred to Ahvaz hospitals and gastroenterology clinics.
The IBS patients were diagnosed based on the obtained information about the GI symptoms in the last 3 months and physical examination using the Rome III criteria. Other cases were consisted of individuals with an inflammatory bowel disease (IBD), colon polyps, hemorrhoids, constipation, and diarrhea. The control subjects were selected from healthy population referred to health centers for check-up and were without any history of GI diseases. All fresh fecal samples were placed in stool containers and were transported to the department of parasitology, Ahvaz Jundishapur University of Medical Sciences. Exclusion criteria for both case and control groups were presence of intestinal parasites other than Blastocystis, bacterial or viral diarrhea, surgery, cancer, radiotherapy, chemotherapy, and treated with antibiotic during the last month.
3.3. Microscopy
Collected samples were examined by light microscopy before culture using Lugol’s stained smears at 100× and 400× magnification to detect Blastocystis.
3.4. Culture
From each fecal sample, approximately 100 mg was inoculated into 4 mL of monophasic Jones’ medium supplemented with 10% inactivated horse serum and incubated at 37 °C. The sediment was examined microscopically with the Lugol’s stain after 48 and 72 hours at 100× and 400× magnification. Positive samples were passaged into fresh medium for another 3 days, then 200 µL of the pellet of each positive sample was transferred to 2 mL tubes and stored at -20°C until examination (1, 20).
3.5. DNA Extraction
Genomic DNA was extracted from 200 µL of the pellet using the QIAamp DNA Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions with slight modifications in the first and final steps. The cultured positive samples were washed three times in a sterile PBS buffer and then were placed in water bath for 10 minutes at 100°C. The DNA extraction was continued according to the manufacturer’s protocol and in the final step, an incubation time was increased to five minutes to improve the yield of DNA (21). Afterwards, DNA extracts were stored at -20°C until the polymerase chain reaction (PCR).
3.6. PCR Amplification
The subtypes of Blastocystis samples were identified with PCR amplification of the small subunit ribosomal RNA genes (SSU-rDNA) using the primers BhRDr (5’-GAG CTT TTT AAC TGC AAC AAC G-3’), RD5 (5’-ATC TGG TTGATC CTG CCAGTA-3’), and conditions as previously described with slight modifications (1). The PCR was performed in a 20 µL volume reaction containing 10 µL of Taq DNA Polymerase 2× Master Mix RED (Ampliqon-Biomol, Hamburg, Germany), 3 µL H2O, 2 µL DNA template, and 2.5 µL of each primer (1.25 µm concentration). The amplified 600 bp fragments of the SSU-rDNA of Blastocystis were visualized on a UV transilluminator after electrophoresis in 1.5% agarose gels and staining with ethidium bromide.
3.7. Sequencing
All PCR products were purified using the MinElute PCR purification Kit (Qiagen, Germany) according to the manufacturer’s protocol and directly sequenced at the Bioneer Co. (Daejeon, South Korea) in one direction using the BhRDr primer. The obtained nucleotide sequences were edited using MEGA 6.0 and aligned with ClustalW, and then compared with published sequences of Blastocystis available in the GenBank database (ncbi.nlm.nih.gov/BLAST).
3.8. Statistical Analysis
Statistical analysis was conducted using PASW 18 (IBM, Armonk, NY, USA), and the prevalence rates were compared between the case and control groups by an Independent samples t test and Chi-square testing.
4. Results
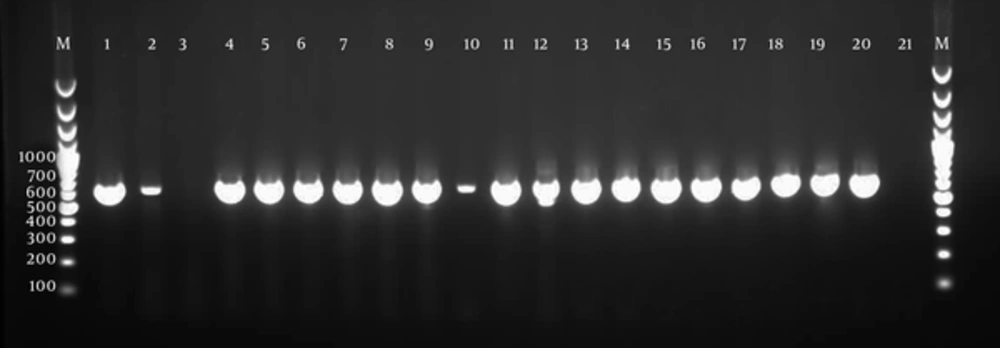
Of the 282 samples included in the current study, 18 samples (6.4 %), including two (1.3%) of the case group patients, and 16 (12.3%) of the control group were positive by light microscopy for Blastocystis. Stool culture was positive in 16 cases (15 with IBS and 1 without IBS) and 40 control samples. From the 56 culture-positive samples, the expected 600 bp fragments of the SSU-rDNA gene were identified in 55 samples (Figure 1). One sample, which was negative by microscopy, was positive in culture, but negative by PCR analysis. The small amount of DNA is probably the cause of negative result by PCR. Among the PCR-positive samples, 15 (27.3%) were cases and 40 (72.7%) were controls. Significant differences were found between the case and control groups.
All PCR-positive samples were sequenced, however 54 samples were successfully sequenced, and DNA sequencing of the one PCR-positive sample from the control group could not be interpreted (Table 1). The obtained sequences identified STs 1, 2, and 3 in the current study. No other STs of Blastocystis and coinfections of subtypes were identified. The most common ST was ST3 with the frequency of 46.3%, followed by ST2, 37% and ST1, 16.7%. A significant difference was found between the studied groups and Blastocystis subtypes (P = 0.000) (Table 2). The obtained sequences have been deposited in the NCBI GenBank database with the accession numbers LC175853-LC175906 for the SSU-rDNA gene of Blastocystis. All isolates showed 99% or 100% identity with sequences in the GenBank.
| Test | Cases | Controls | Total | |
|---|---|---|---|---|
| With IBS | Without IBS | |||
| Microscopy | 2/105 (1.9) | 0/47 (0) | 16/130 (12.3) | 18/282 (6.4) |
| Culture | 15/105 (14.3) | 1/47 (2.1) | 40/130 (30.1) | 56/282 (19.9) |
| PCR | 14/105 (13.3) | 1/47 (2.1) | 40/130 (30.1) | 55/282 (19.5) |
Abbreviations: IBS, Irritable Bowel Syndrome; PCR, Polymerase Chain Reaction.
aValues are expressed as No. (%).
| Subtypes | Cases | Controls | Total | |
|---|---|---|---|---|
| With IBS | Without IBS | |||
| ST1 | 2 (3.7) | 0 (0) | 7 (13) | 9 (16.7) |
| ST2 | 6 (11.1) | 1 (1.9) | 13 (24) | 20 (37) |
| ST3 | 6 (11.1) | 0 (0) | 19 (35.2) | 25 (46.3) |
| Total | 14 (25.9) | 1 (1.9) | 39 (72.2) | 54 (100) |
Abbreviations, IBS, Irritable Bowel Syndrome; ST, Subtype.
aValues are expressed as No. (%).
The distribution of STs in the age groups was summarized in Table 3. The highest frequency of Blastocystis STs (27.8%) was identified in the age group of 31 - 40 and the lowest was found in the age groups of under 10 years and over 81 years. Of the 54 detected STs, ST2 (3.7%) was the only ST found in the age group of 71 - 80. No significant association was found between the age groups and distribution of STs (Table 3). The prevalence of Blastocystis in males was higher (69.1%) than females (30.9%), but no significant correlation was found between infection and sex. Among the 47 patients without IBS, 55.3% were males and 44.7% were females. Blastocystis infection (ST2) was only detected in one (1.9%) patient with diarrhea (Table 4).
| Age Group, y | Blastocystis Subtypes | Total | |||||
|---|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | |||||
| Case | Control | Case | Control | Case | Control | ||
| 11 - 20 | 0 | 0 | 2/16 (12.5) | 0 | 1/16 (6.2) | 0 | 3/16 (18.7) |
| 21 - 30 | 0 | 2/55 (3.7) | 0 | 6/55 (10.9) | 2/55 (3.7) | 4/55 (7.2) | 14/55 (25.5) |
| 31 - 40 | 0 | 2/73 (2.7) | 2/73 (2.7) | 4/73 (5.5) | 1/73 (1.4) | 6/73 (8.2) | 15/73 (20.5) |
| 41 - 50 | 0 | 1/59 (1.7) | 0 | 2/59 (3.4) | 1/59 (1.7) | 6/59 (10.2) | 10/55 (17) |
| 51-60 | 1/31 (3.2) | 2/31 (6.5) | 1/31 (3.2) | 1/31 (3.2) | 0 | 2/31 (6.5) | 7/31 (22.6) |
| 61 - 70 | 1/29 (3.4) | 0 | 0 | 0 | 0 | 2/29 (6.9) | 3/29 (10.3) |
| 71 - 80 | 0 | 0 | 2/8 (25) | 0 | 0 | 2/8 (25) | |
| Total | 2/152 (1.3) | 7/130 (5.4) | 7/152 (4.6) | 13/130 (10) | 5/152 (3.3) | 20/130 (15.4) | 54/282 (19.1) |
aValues are expressed as No. (%).
| Disease | No. | Male | Female | Blastocystis |
|---|---|---|---|---|
| Dysentery | 18 (6.4) | 10 (21.3) | 8 (17) | 0 |
| Colonic polyps | 2 (0.7) | 1 (2.1) | 1 (2.1) | 0 |
| Diarrhea | 11 (3.9) | 4 (8.5) | 7 (14.9) | 1 (1.9) |
| IBD | 1 (0.4) | 1 (2.1) | 0 (0) | 0 |
| Melena | 7 (2.5) | 6 (12.8) | 1 (2.2) | 0 |
| Rectorrhagia | 2 (0.7) | 1 (2.1) | 1 (2.1) | 0 |
| Colitis | 2 (0.7) | 0 (0) | 2 (4.3) | 0 |
| Others | 4 (8.5) | 3 (6.4) | 1 (2.1) | 0 |
| Total | 47 (100) | 26 (55.3) | 21 (44.7) | 1 (1.9) |
aValues are expressed as No. (%).
5. Discussion
Blastocystis is an intestinal parasite of both humans and animals with a controversial pathogenic role (7). Since Blastocystis has been linked to the IBS syndrome, various studies have focused on this concern around the world (22). Reliable data on the prevalence and diversity of Blastocystis in the IBS patients in Iran are scarce and restricted to a few studies (18, 23). In our knowledge, this study is the first report of the frequency of Blastocystis subtypes in patients with GI symptoms, particularly IBS patients in comparison with healthy people from the southwest of Iran. We expected that Blastocystis would be more prevalent in patients with GI symptoms than in the control group, but the observed prevalence rates, including 13.3% in subjects with IBS and 2.1% in patients without IBS were lower than the control group (30.1%) (Table 1).
Our results are in contrast with a study from the west of Iran (23) and other studies from Turkey, India, France, and Mexico which showed a higher prevalence of Blastocystis infection in IBS population compared to the control group (2, 6, 24, 25), but similar to those reported studies from Thailand and Denmark (26, 27). A notable feature of the current study is that the control group was selected from healthy people without GI symptoms, which it can be extended to the whole community in Ahvaz. In the present study, ST3 was the most common detected ST, followed by STs 2 and 1 (Table 2). Although distribution of the Blastocystis STs varies from country to country and between different regions of the country (1), the subtype 3 is the most common subtype in humans around the world (28). Two studies from Pakistan and Egypt showed that ST1 is the predominant ST in IBS patients (29, 30) , while from UK and France STs 3 and 4 were reported as the most common STs in IBS patients (1, 2). Despite the fact that STs 3 and 1 have been described with more pathogenicity (31, 32), we found a higher infection rate of these STs in asymptomatic subjects (the control group) (Table 2).
There is some evidence showing that occurrence of ST2 in Australia, Africa, and East Asia is rare, but in our study, ST2 was the second most prevalent subtype identified in IBS patients and controls (1). Difference in the diversity and prevalence of STs is probably due to different factors such as cultural behaviors, geographical location, temperature, exposure to reservoir hosts, and transmission routs (24). While, Blastocystis STs is reported in different countries with a different prevalence, subtype 4 has limited distribution and is common in Europe. Thus, it is rarely reported from other countries such as Asian, Middle Eastern, and South American countries (1, 12). One study from Turkey reported ST4 in one patient with abdominal pain (33). Another study from Italy ST4 was detected in 21.7% of patients with IBS and IBD (34). Furthermore, Forsell et al. from Sweden highlighted ST4 in 20.6% of examined patients in the Stockholm area (7). In our study, ST4 was not found. It is likely that, the absent of ST4 in our study is due to rare infection of this ST in subtropical countries (1).
In the present study, the higher prevalence of Blastocystis (27.8%) observed at the age group of 31 - 40 and the lower prevalence (0%) at the age groups under 10 and above 80 years (Table 3). Due to the small sample size in these age groups, it is difficult to demonstrate a significant difference between age and infection. However, two studies from Ireland and India indicated that frequency of Blastocystis in children is lower than adults (35, 36). The higher exposure to the parasite possibly increases the risk of infection with increasing age (22). The frequency of Blastocystis in males was two times than females and ST3 was the most common ST in them. A study by Forsell et al. (2012) indicated that ST3 was more frequent in males compared to females (7). In another study on the IBS patients, Blastocystis was more common in males in the control group (2). Although, the higher number of males, particularly in the control group makes interpretation of our results more difficult, it seems that more contact to the parasite in males is probably the cause of this increase (7). Therefore, more studies should investigate whether sex is associated with infection.
The higher prevalence of Blastocystis in asymptomatic individuals (healthy groups) develops the hypothesis of Blastocystis colonization without development of symptoms (4). The supporting evidence is that we found Blastocystis in 14/105 cases with IBS and only one case without IBS, while 39/130 healthy people were infected. The only positive sample of the case group without IBS belonged to a 75-year-old man who was hospitalized due to diarrhea. In two IBD patients, we could not find Blastocystis infection. A previous study investigated Blastocystis infection in Danish patients with IBD compared to healthy controls, which controls showed higher infection (19%) than cases (5%) (37). In recent years, many researches have been performed to elucidate the possible role of Blastocystis in health or disease. In many studies it was linked to bowel diseases (27, 37-39), but recent studies show that this organism might have an important role in human health and could be considered as a gastrointestinal health marker (5).
The inverse relationship between Blastocystis colonization and bowel diseases has been proposed recently. The gut microbiota in patients who affected by intestinal diseases such as IBS and IBD are different from healthy people’s guts (5). Thus, our data are in agreement with the mentioned hypothesis and lower occurrence of Blastocystis in the case group compared to the healthy group could be attributed to the intestinal bacterial profiles. Although, the possible role of some intestinal protozoa such as Blastocystis, G. duodenalis and Entamoeba histolytica has been defined in the etiology of IBS (10), our finding is presumably a consequence of changes in the bacterial flora of intestinal infection of the subjects. Mixed infection with STs was not observed in this study.
In our knowledge, this is the first and one of the few conducted molecular studies on Blastocystis STs in IBS patients from the Khuzestan Province, southwest Iran. Due to an unexpectedly higher prevalence of Blastocystis among healthy people in this study, We could not find significant associations between Blastocystis and the IBS disease, but the obtained data support the hypothesis that Blastocystis might be a GI health marker, however, further studies in different population of healthy and patients in different urban and rural regions of the country are necessery to elucidate the posible role of the parasit in disease or health.