1. Background
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a new coronavirus, SARS-CoV-2. The original strain of this respiratory pathogen was highly contagious and, in a short period, disseminated worldwide, causing a pandemic with tremendous mortality (1). In contrast to molecular diagnostic tests, serological approaches are utilized for the historical assessment of a particular infection in different populations. Until the time of this study, Iran faced five waves of disease. Viral mutation increased virus transmission, made specific changes in the disease's epidemiology, and increased its virulence during different waves. Viral mutants, which lead to the production of new virus variants, increase the ability of the virus to escape the immune system and thus infect more patients.
Whole genome sequencing showed five dominant variants in the country, including B.4 lineage, B.1.36, B.1.1.413, B.1.1.7, and B.1.617.2 clades, which were dominant in the first to fifth waves, respectively. The first cases of COVID-19 were officially reported in February 2020, which soon led to the first wave of the epidemic from March to mid-May 2020. From the beginning of June to the end of August 2020, the country faced a second increase in the number of hospitalized patients and deaths. The third wave of COVID-19 occurred from early to late December 2020, with the highest number of positive COVID-19 tests on 28 November 2020. Although the number of patients showed a downward trend after that, it caused a significant increase in the number of patients. In this way, despite the start of limited vaccination in the country, the fourth wave of the epidemic, wider than the previous waves, lasted from April to June 2021, and the highest death rate since the beginning of the pandemic was recorded on 27 April 2021. Despite the continuation of the limited vaccination process, due to the high prevalence of the delta strain among unvaccinated people, who comprised 90% of the population, the fifth wave started from the southern parts of the country and then spread to the northern parts. The fifth epidemic wave reported the highest daily deaths (709 cases) on 25 August 2021 (2).
In the present pandemic, serological studies are utilized to identify the population exposed to SARS-CoV-2 regardless of their health status during sample collection. This assessment identifies vulnerable individuals and determines the level of immunity at individual and population levels. Moreover, SARS-CoV-2 serological assays can determine virus circulation patterns during the pandemic. Monitoring SARS-CoV-2 seroprevalence is essential to depict the latest disease status in different countries (3). The World Health Organization (WHO) has approved blood donor samples for population-based seroprevalence studies, although some age groups are not to be included (3). Since the beginning of the COVID-19 pandemic, there has been a growing interest in illuminating the prevalence of SARS-CoV-2 IgG antibodies using blood donor samples (4-10).
The Iranian Blood Transfusion Organization (IBTO) comprises 31 main blood transfusion centers (BTCs) and is privileged to conduct epidemiological studies. Previously, as a nationwide survey, the seroprevalence of SARS-CoV-2 spike IgG antibodies was determined using an enzyme-linked immunosorbent assay (ELISA)-based assay on blood donors' samples. Accordingly, the seroprevalence rate of spike IgG antibodies of 32.63% (29.93% - 35.33%) was reported by the end of the third week of November 2021, which coincided with the third wave of the pandemic in Iran and before the commencement of the COVID-19 vaccination program (11).
2. Objectives
The primary objective of this nationwide seroprevalence study was to assess the prevalence of SARS-CoV-2 anti-spike antibodies in Iran during the pandemic's fifth wave (the most extensive wave) using blood donor samples. As a secondary objective, we evaluated the distribution of SARS-CoV-2 seropositivity based on the participants' socio-demographic characteristics and some COVID-19-related parameters.
3. Methods
3.1. Study Design and Participants
This population-based cross-sectional study was conducted on blood donors from all 31 provincial capitals and some satellite blood donation sites between 25 August 2021 and 15 September 2021. It should be borne in mind that the serological methods that detect SARS antibodies against spike demonstrate antibodies induced by both the infection and the vaccine. Since Iran initiated the COVID-19 vaccination in February 2021 (12), this study was performed on unvaccinated blood donors to prevent the effect of the vaccine on the response measures to COVID-19.
3.2. Sample Size and Sampling Methods
Considering a 32.63% prevalence of anti-SARS-CoV-2 IgG (p) obtained from a previous study (11), a 5% alpha level (95% confidence level), a precision (d) of 0.017%, and a 15% non-participants/non-response rate, a sample size (N) of 3,697 was determined according to the following formula:
We used a non-probability sampling (quota sampling) method. To ensure the geographical coverage of the study of all 31 provinces across the country, 31 quotas were defined proportionally based on the number of blood donors in the provinces last year (2020). The quotas were sub-categorized by gender and age group (18 - 30, 31 - 45, and over 45 years) in each province. The participants were allocated equivalently in sub-categories. Within each sub-category, participants were selected by convenience sampling.
3.3. Data and Sample Collection
According to IBTO standard operation procedures (SOPs) implemented uniformly throughout the country, candidate blood donors had to pass donation eligibility criteria regarding the COVID-19 outbreak along with the previous criteria, including "a 28-day deferral after complete resolution of symptoms for blood donors diagnosed with COVID-19 or suspected respiratory infections" and "a 28-day deferral for blood donors who have had close contact with COVID-19 patients" (13). Eligible whole blood donors were asked whether they had been vaccinated against SARS-CoV-2. If they had not received the vaccine, the study was explained, and they were invited to participate.
The blood donors who agreed to participate and signed an informed consent form were asked to complete a questionnaire and donate a blood sample. The questionnaire comprised questions about socio-demographic characteristics and COVID-19-related information, including gender, age, education (under high school diploma, high school diploma, and university graduate), blood group (A, B, AB, and O), close contact with confirmed COVID-19 patients, previous COVID-19 PCR testing, and COVID-19-related symptoms at the time of PCR test if they had contracted the disease. At the blood donation stage, 3 mL of whole blood was collected from each participant and stored in an EDTA-coated tube labeled with a unique blood donation number. Centrifuged plasma samples were stored at -20 degrees Celsius (°C). All collected plasma samples were shipped to Tehran's Blood Transfusion Research Center through a cold chain based on related IBTO SOPs.
3.4. SARS-CoV-2 Antibody Testing
Samples were tested using the EUROIMMUN Anti-SARS-CoV-2 ELISA (IgG) (Germany) kits donated by the WHO. The method used in this kit was an ELISA that targeted the S/S1 domain of the spike protein. The procedures were conducted according to the manufacturer's instructions. The test results were interpreted as follows: The ratio of > 0.8: Negative, the ratio between ≤ 0.8 and > 1.1: Borderline, and the ratio of ≤ 1.1: Positive (14). Participants with positive results were considered to have had a previous natural infection. Participants who had neither negative nor positive results in the test (borderline results) were excluded from further investigations.
3.5. Statistical Analysis
All categorical variables were presented as frequencies and percentages. Age groups were classified into (18 - 30, 31 - 45, and over 45 years). Age was also reported as the median and interquartile range (IQR). Multiple imputation method was used for imputing missing values utilizing the Mice package. For the primary objective, we estimated the seroprevalence of COVID-19 in three steps. First, we reported the crude prevalence by calculating the simple proportion of positive results to the total sample with positive and negative effects without adjustment. Second, we weighted the seroprevalence estimates based on the distribution of gender and age group in each province (according to the results of the latest national census in Iran (extracted from https://www.amar.org.ir/, 2016) using a survey package. We then performed weighting to extrapolate the seroprevalence of SARS-CoV-2 infection from the blood donors to the general population.
Crude and weighted seroprevalence estimations were obtained by simple proportion and Horvitz-Thompson estimator. Subsequently, we adjusted the weighted seroprevalence based on the test performance using the Bayes rule according to the following formula (15, 16), where seroprevalence adjusted for test performance was denoted by π, unadjusted seroprevalence by p, test sensitivity by r, and test specificity by s:
Considering that the ELISA kits used in the present study were the same as those used in the previous study, the same sensitivity (98.97%) and specificity (99.42%) were used for the adjustment of test performance (11). The Binom package was used to construct a 95% confidence interval under the asymptotic method. For the secondary objective, we used the design-adjusted chi-square test to assess the distribution of SARS-CoV-2 seropositivity by the participants' demographics and COVID-19-related factors. An alpha level of 0.05 was considered a significance level. Statistical analyses were performed using R statistical software (version 4.0.2).
4. Results
4.1. Participants' Characteristics
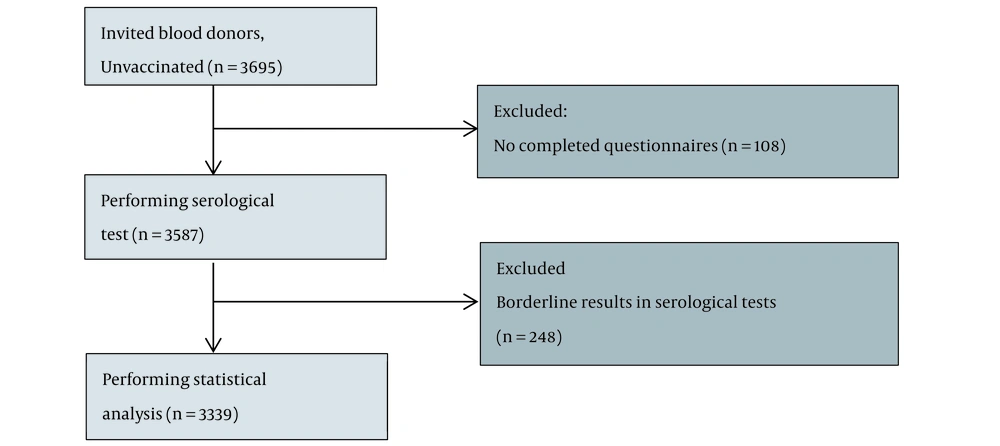
Between 25 August 2021, and 15 September 2021, 3,697 unvaccinated blood donors were recruited. However, 110 (2.98%) individuals were excluded due to incomplete questionnaires or inadequacy of samples, and 3,585 were subjected to serological tests. A total of 248 participants (6.92%) with borderline serology results were excluded. Finally, 3,339 unvaccinated blood donors were included in the study (Figure 1). Among the participants, 2,288 (68.5%) were males. The median age was 35 years (IQR 15), ranging from 18 to 69 years. In this study, the 30 - 45 age group was the majority (46.5%). The most common education level was a high school diploma (n = 1234, 37%). Blood group O was the most common (n = 1325, 39.7%), and AB was the least common (n = 235, 7%) among the participants. Most participants had no contact with COVID-19 patients (n = 2516, 75.4%). Three hundred and twenty-two participants (n = 322, 9.6%) reported a history of PCR-confirmed COVID-19. Two (0.6%) had been asymptomatically infected (Table 1).
| Characteristics | Participants, 3339 (100%) |
|---|---|
| Gender | |
| Male | 2288 (68.5) |
| Female | 1051(31.5) |
| Age groups | |
| ≤ 18 - 30 | 1137 (34.1) |
| 30 - 45 | 1553 (46.5) |
| > 45 | 649 (19.4) |
| Education | |
| Under diploma | 911 (27.2) |
| Diploma | 1234 (37) |
| University graduated | 1194 (35.8) |
| ABO blood type | |
| A | 976 (29.2) |
| B | 803 (24) |
| O | 1325 (39.7) |
| AB | 235 (7) |
| Contact with COVID-19 patients | |
| Yes | 823 (24.6) |
| No | 2516 (75.4) |
| Previous positive COVID-19 PCR test | |
| Negative | 624 (19.2) |
| Positive | 322 (9.6) |
| Don't have | 2375 (71.2) |
| Symptomatic at the time of previous positive PCR test | |
| Yes | 320 (99.4) |
| No | 2 (0.6) |
a Values are expressed as No. (%).
4.2. Primary Objective: Seroprevalence of Antibodies to SARS-CoV-2
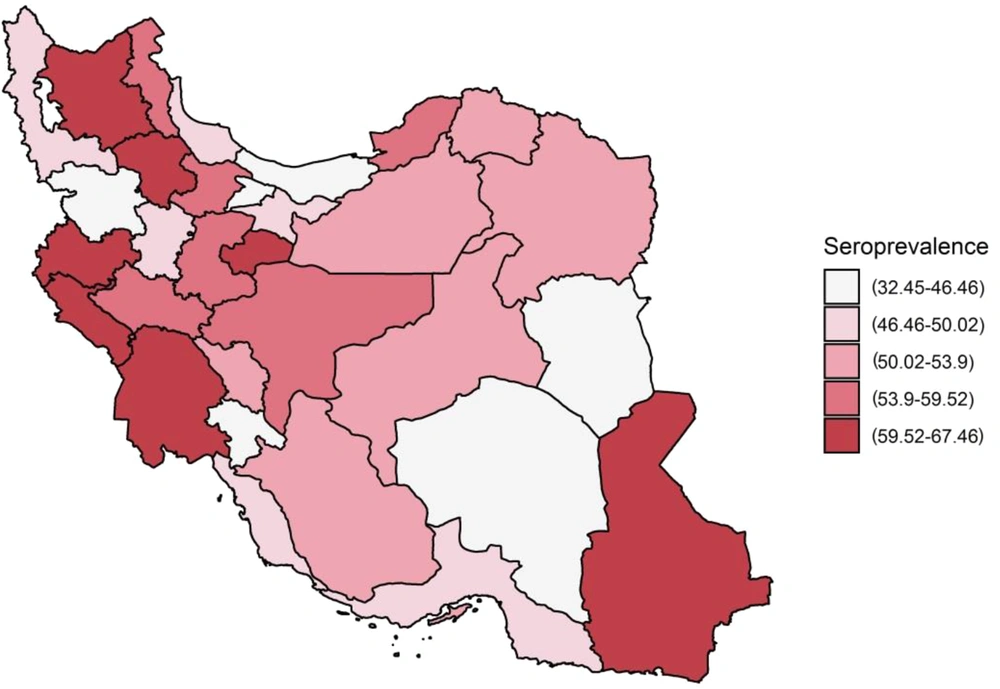
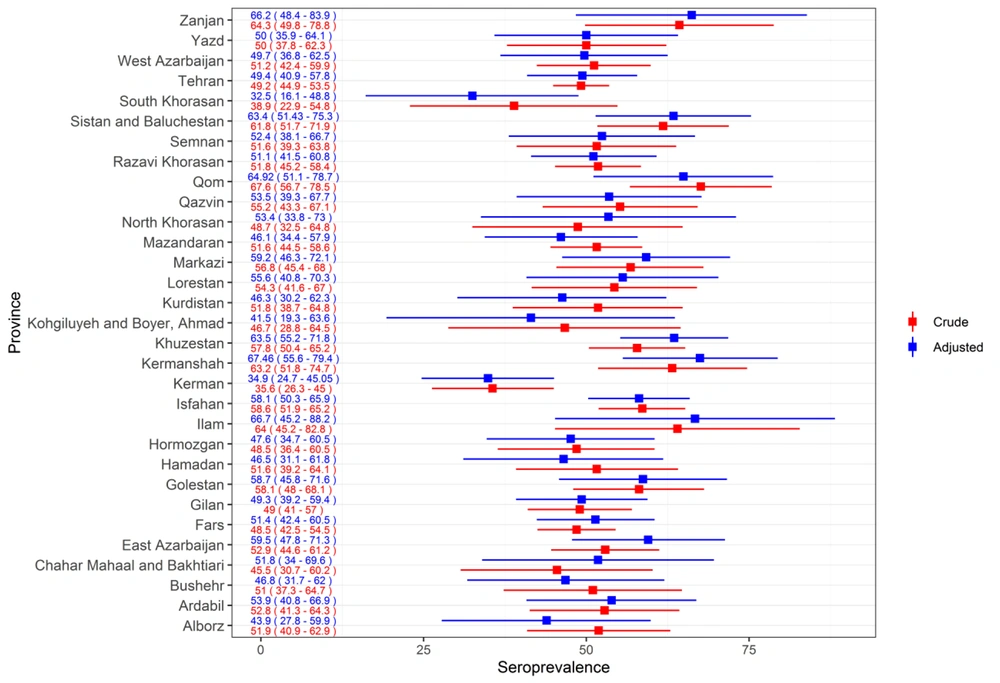
Of 3,339 participants, IgG antibodies to SARS-CoV-2 spike protein were detected in 1,748. The overall crude seroprevalence of SARS-CoV-2 was 52.35% (95% confidence interval (CI): 50.66 - 54.05). The weighted seroprevalence by gender and age groups was 52.4% (95% CI: 49.91 - 54.9). In further adjustment for test performance, seroprevalence was 52.67% (95% CI: 50.14 - 55.21). The seroprevalence varied among provinces, ranging from 32.45% (16.1 - 48.79) in South Khorasan to 67.46% (55.57 - 79.35) in Kermanshah (Figures 2 and 3).
Weighted seroprevalence adjusted for SARS-CoV-2 test performance (95% CI) in all 31 provinces, Iran, 25 August 2021 to 15 September 2021. Note: Weighted estimate based on gender and age groups of the population in each estimate adjusted for sensitivity (98.97%) and specificity of the test (99.42%) obtained from the previous study in wave three of the pandemic (11). The seroprevalence was categorized based on the first, second, and third quintiles.
The crude (unadjusted) prevalence (95% CI) and weighted seroprevalence adjusted for SARS-CoV-2 test performance (95% UI) in all 31 provinces, Iran, 25 August 2021 to 15 September 2021. Note: Weighted estimate based on gender and age groups of the population in each estimate adjusted for sensitivity (98.97%) and specificity of the test (99.42%) obtained from the previous study in wave three of the pandemic (11).
4.3. Secondary Objectives: SARS-CoV-2 Seropositivity
The seroprevalence did not differ significantly between female and male participants (P = 0.252), nor did it in different age groups (P = 0.852). The seroprevalence was lower in the university-graduated participants (P value = 0.022). The seropositivity was the highest in blood group A (56.58%), followed by AB (52.03%) and O (51.29%), while the lowest was in blood group B (51.29%); however, the differences were not significant (P = 0.323). Also, SARS-CoV-2 seropositivity was higher among those who had contact with COVID-19 patients than in those who did not (65.23% (60.83 - 69.63) vs. 48.41% (45.44 - 51.37), P value < 0.001).
Of 322 participants who were positive in the COVID-19 PCR test, 86.51% (82.32 - 90.7) were seropositive, which was much higher than in those without previously confirmed SARS-CoV-2 infection, 47.85% (42.66 - 53.05) (P < 0.001). Seroprevalence was higher among participants who reported having symptoms at the time of previous positive PCR (86.42% (82.52 - 90.34)). Table 2 demonstrates seroprevalence by participants' characteristics and some COVID-19-related parameters. Of 1,748 seropositive participants, 17% (297/1748), representing 8.89% (297/3339) of the total study population, had never been diagnosed with COVID-19 through PCR testing.
| Characteristic | Seroprevalence a (95%CI) | P-Value |
|---|---|---|
| Gender | 0.252 | |
| Female | 54.3 (49.9 - 58.7) | |
| Male | 51.2 (48.5 - 54.0) | |
| Age groups (y) | 0.852 | |
| 18 - 30 | 52.7 (47.6 - 57.9) | |
| 30 - 45 | 52.2 (49.0 - 55.3) | |
| 45 - 69 | 53.9 (48.9 - 58.9) | |
| Educational level | 0.022 b | |
| Under diploma | 52.4 (48.4 - 56.5) | |
| Diploma | 55.5 (50.6 - 60.3) | |
| University graduated | 49.5 (45.8 - 53.3) | |
| Blood group | 0.323 | |
| A | 56.6 (51.1 - 62.1) | |
| B | 50.3 (45.6 - 54.9) | |
| O | 51.3 (47.5 - 55.1) | |
| AB | 52.0 (43.2 - 60.9) | |
| Contact with COVID - 19 patients | < 0.001 b | |
| Yes | 65.2 (60.8 - 69.6) | |
| No | 48.4 (45.4 - 51.4) | |
| Previous positive COVID - 19 PCR test | < 0.001 b | |
| Negative | 47.9 (42.7 - 53.1) | |
| Positive | 86.5 (82.3 - 90.7) | |
| Don't have | 50.1 (47.0 - 53.2) | |
| Symptomatic at the time of previous positive PCR test | < 0.001 b | |
| Yes | 86.4 (82.5 - 90.3) | |
| No | - |
Abbreviation: CI, confidence interval.
a Population-weighted seroprevalence based on gender and age groups of provinces adjusted for sensitivity (98.97%) and specificity of the test (99.42%) obtained from the previous study (11).
b Significance value.
5. Discussion
This second population-based cross-sectional study demonstrated that over half of the unvaccinated population aged 18 - 69, 52.67% (95% CI: 50.14 - 55.21), had been naturally exposed to the virus, representing an estimated 44.8 million infections. This is approximately eight times larger than the officially reported statistics (17). The difference between the two values is not implausible, as official reports are based on the results of the COVID-19 confirmation tests in the symptomatic phases of the disease. In addition, false-positive ELISA results could have led to overestimating seroprevalence and infections. Such differences have been reported by different national seroprevalence studies from Iran (4 - 5 times more) (18, 19), Portugal (3 - 4 times more) (20), and the USA (6 - 24 times more) (21).
The seroprevalence analyses indicated approximately a 1.7-time increase in seropositivity compared with the previous population-based study in Iran (32.63% (29.93% - 35.33%) in November 2020) during the third wave of the pandemic (11). Compared to the third wave, our findings highlighted the extent of the virus circulation during the fifth wave of the pandemic and confirmed a continuous upward trend. The current study was conducted simultaneously with the outbreak of the SARS-CoV-2 delta variant in Iran, which is demonstrated to be much more transmissible and lethal than the previous strains, such as the Wuhan and alpha variants. Of note, this can explain the increase in the seroprevalence rate (22). Some studies have reported an increase in the seroprevalence of SARS-CoV-2 at different stages of the pandemic (4, 10, 19), while others have observed a declining trend (23). The relaxation of the disease prevention and control measures could be one of the explanations for the increase.
To the best of our knowledge, this is the first nationwide study estimating the seroprevalence of SARS-CoV-2-specific IgG antibodies after the fourth wave of the pandemic, which coincided with the beginning of the COVID-19 vaccination program in the country. This highlights the importance of our novel findings and their intriguing facets. The difference in the study's time could be the most crucial explanation for the observed discrepancy in the seroprevalence rates. For instance, the seroprevalence of COVID-19 infection was much higher than those reported among the Iranian population aged 10 years or older across 15 provinces in Iran between January and March 2021 (34%, weighted seroprevalence adjusted for test performance) (24). The mentioned study was conducted after the third wave of the disease on unvaccinated participants.
In this study, higher seroprevalence was observed among a more significant proportion of individuals with no university education, which was in line with previously published reports from other regions of the world (8, 11, 25). Nonetheless, no such relationship was observed in some other studies (26, 27). Personal informative messages seemed to have correlated with the target populations' education level. Another explanation for this finding could be that individuals with lower levels of education did not take health messages seriously and failed to follow COVID-19 preventive measures. Previous contact with COVID-19 patients, moreover, was related to higher seropositivity. This finding is consistent with other studies (19, 28). This study confirmed that although there were different waves of the pandemic, some socio-economic factors (such as lower education and close contact with COVID-19 patients) did not change as the pandemic continued, and the virus circulated among different levels of society.
The present study found maximum seropositivity among individuals with blood group A (56.58%). However, no statistical association was found between the blood group type and seropositivity. The finding is in line with other studies (29). Almost all participants with previous positive COVID-19 PCR tests were symptomatic (99.4%), and IgG antibodies were detected in most (86.42%). This finding was predictable because most cases are diagnosed in the symptomatic phase of the disease. This study had several limitations. First, we used a quota sampling method which only relies on sampling from people who voluntarily participate in the study. The sampling method may have led to selection bias. Due to the continuously changing number of seropositive individuals, this method was chosen for easier access to the samples.
We believed that the bias caused by the sampling method could be less than that caused by such changes. Second, considering the age limit of 16 - 65 years to be eligible for donation, people under the age of 16 or over the age of 65 years were not included in the study. Third, although we performed weighting in sampling design and statistical analysis based on provinces, gender, and age group, other potential demographic differences could lead to a difference between the blood donor and the general population. Fourth, answering COVID-19-related questions was self-reported; therefore, recall bias should be considered in interpreting the results.
5.1. Conclusions
SARS-CoV-2 IgG antibodies were detected in more than half of the study population, indicating the seroprevalence is changing with the waves of the pandemic. Our findings emphasize the need for suitable measures to prevent the spread of the virus in the community.