1. Background
Chronic kidney disease (CKD) is one of the most significant health concerns in every country, and CKD patients constitute a significant portion of every society, with a global prevalence of 13.4% (11.7 - 15.1%) (1). End-stage renal disease (ESRD) is the final and permanent stage of CKD in which kidney function decreases to the point where the kidneys can no longer function independently. The majority of patients with ESRD require dialysis or a kidney transplant. Consequently, 71% and 29% of ESRD patients in the United States require dialysis and a kidney transplant, respectively (2). As a widespread treatment method for ESRD patients, dialysis removes excess water, soluble substances, and toxins from the blood, performing a function similar to a healthy kidney (3). Multiple risk factors have been reported to predispose individuals to CKD or the progression of the disease, the most significant of which are African-American race, male gender, age over 60, positive family history, smoking, obesity, underlying diseases like diabetes mellitus (DM) and hypertension, and infectious agents like hepatitis B virus (HBV), human immunodeficiency virus (HIV), human T-lymphotropic virus 1 (HTLV-1), and hepatitis C virus (HCV) (4-6).
As one of the most considerable infectious agents in hemodialysis patients, HCV has been reported to have a varying prevalence in hemodialysis patients in different regions of the world, ranging from 1% in Europe to 85% in South Africa (7). As known, HCV, a Flaviviridae family member belonging to the Hepacivirus genus, causes non-A, non-B hepatitis. It is subdivided into at least 8 genotypes and more than 86 subtypes based on the difference in genome sequence (8, 9). The frequency of these genotypes varies, with genotype 1 (49.1%), genotype 3 (17.9%), genotype 4 (16.8%), and genotype 2 (11.0%) being the most common, and genotypes 5, 6, 7, and 8 having the lowest frequency (6%) (10). The HCV genome is single-stranded RNA with a positive sense that encodes 10 structural and non-structural proteins (8).
A new type of chronic HCV infection known as occult hepatitis C infection (OCI) was discovered by Castillo and colleagues about 18 years ago. It is characterized by the presence of HCV-RNA in hepatocytes or peripheral blood mononuclear cells (PBMCs), while serum HCV-RNA is negative, and anti-HCV is positive or negative. In other words, OCI is the presence of HCV infection without viremia or serological symptoms (11, 12). Since HCV-RNA can infect many types of PBMC, including B cells, T cells, monocytes, and dendritic cells, the HCV replication in PBMCs may be the source of HCV reinfection after antiviral treatment or liver transplantation, and these cells are thought to be the main extrahepatic reservoir for HCV (13). The prevalence of OCI in hemodialysis patients varies by country, ranging from 0% to 49% (14).
2. Objectives
Although OCI infection is less severe than chronic hepatitis C infection, it can eventually lead to liver cirrhosis, an important leading cause of liver-related mortality, liver failure, and hepatocellular carcinoma (HCC) (15, 16). As there is a lack of studies on the diagnosis and treatment of this infection in Iran, we decided to investigate the prevalence of HCV and OCI infections in hemodialysis patients in Kerman. This research addresses the need for attention to diagnosing and treating these infections.
3. Methods
3.1. Study Design
In this cross-sectional study, patient information and samples were collected for 4 months from December 2021 to March 2022 from Javad-Al-Aemeh Clinic, one of the dialysis centers in Kerman city.
3.2. Exclusion and Inclusion Criteria
Inclusion criteria included CKD patients with a history of dialysis within the past six months who were fully alert and consented to the study. Exclusion criteria included patients with a history of other chronic liver diseases, such as alcoholic liver disease or autoimmune liver disease, HIV-infected individuals, patients with significant comorbidities, such as advanced cancer or severe heart disease, and those with a history of organ transplantation.
3.3. Patients and Sample Collection
In this study, 100 hemodialysis patients were referred to the Kerman dialysis center for evaluation. After completing a questionnaire containing the patient's demographic information and past medical history, a 10 mL venous blood sample was collected in two tubes, one without anticoagulant (2 mL) and the other with heparin (8 mL). The first tube was used for serology tests, and the other tube was used with heparin anticoagulant to isolate PBMCs.
3.4. Preparation of Specimens
The patient's sera were separated from the tube without anticoagulant by centrifugation at 2,000 rpm for 10 minutes and stored in a freezer at -70°C until the enzyme-linked immunosorbent assay (ELISA) tests. To isolate PBMCs, the tube containing the anticoagulant was diluted with an equal proportion of PBS. Then, it was slowly poured with a Pasteur pipette onto cold Ficoll (equal to the volume of blood) to separate them into two layers. After that, they were centrifuged at 2,500 rpm for 30 minutes. Then, PBMCs were separated in the formed phase. After being washed twice with PBS, they were finally added to the cell precipitate of 500 μL lysis buffer and stored in a -70°C freezer until the molecular test was performed.
3.5. Serological Test
Serum samples from all patients were evaluated by ELISA to examine the presence of anti-HCV antibodies. All tests were conducted according to the manufacturer's recommendation. The test kit was from Pishtaz Teb, Iran.
3.6. RNA Isolation and cDNA Synthesis
Total RNA was extracted from serum samples, and PBMCs of all patients were isolated using the SinaPure viral extraction kit (Sinaclon, Iran) according to the manufacturer's instructions. To evaluate the concentration and purity of the extracted RNA, 1 μL of each sample was measured by a NanoDrop™ 2000 (Thermo Scientific, USA). Additionally, the quality control of RNA integrity was done by running 4 μL of the extracted RNA, along with 2 μL of loading dye, on a 2% agarose gel to observe the bands of 18srRNA and 28srRNA. In the next step, the extracted RNA from each sample was used in the cDNA synthesis reaction. The reaction was carried out using the AddScript cDNA synthesis kit (Add Bio, Korea) following the instructions provided by the manufacturer. In this reaction, 1 to 4 μg (based on concentration) of each sample, along with 1 μL of 50 μM random hexamer, 13.4 μL of diethyl pyrocarbonate (DEPC) water, 1 μL of deoxynucleotide triphosphate (dNTPs) (10 mM), 1 μL of the RNase inhibitor (20 units), and 1 μL of reverse transcriptase (200 units) were prepared in a total volume of 20 μL and incubated according to the temperature program. The cDNA synthesized samples were placed in a -70°C freezer until the molecular step was performed.
3.7. Reverse Transcription-Quantitative Polymerase Chain Reaction and Genotyping
The real-time polymerase chain reaction (PCR) was performed using a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, USA). Also, HCV-specific primers and probes (5'-UTR) were used to check the presence of the HCV genome in the samples (Table 1). The reverse transcription-quantitative polymerase chain reaction (RT-qPCR) mixture in the final volume of 25 µL contained 12.5 µL RealQ Plus 2x Master Mix, 0.5 mM of each primer, 10.5 µL PCR-grade H2O, and 1 µL cDNA. The amplification conditions were 3 minutes at 95°C, 45 cycles of 10 seconds at 94°C, and 40 seconds at 55°C, followed by a melting curve cycle. The β-actin gene as an internal control and the HCV-positive serum sample as a positive control were used in the reaction (Table 1) (17). Then, the HCV-positive samples underwent genotyping using genotype-specific primers, as described previously (Table 1) (2).
| Locus | Oligonucleotide Sequence | Products Size, bp |
|---|---|---|
| HCV-F | CGGGAGAGCCATAGTGGTC | 179 |
| HCV-R | GCAAGCACCCTATCAGGCA | |
| HCV-probe | HEX-CAAGGCCTTTCGCGACCCAAC-BHQ1 | |
| HCV-1 | 5′ HEX- AAG GAC CCG GTC CT 3′ | 128 |
| HCV-2 | 5′ FAM- TAT CCA AGA AAG GAC CCA 3′ | 137 |
| HCV-3 | 5′ FAM- CAA CAC TAC TCG GCT AGT 3′ | 200 |
| HCV-4 | 5′ HEX- CAT GGC GTT AGT ATG AGT GTT 3′ | 229 |
| HCV-1a | 5′ HEX- ACT CGG CTA GCA GTC TT 3′ | 193 |
| HCV-1b | 5′ FAM- ACT CGG CTA GCA GTC TC 3′ | 193 |
| HCV-1c | 5′ FAM- CCG GTT CCG CAG ACC ACT 3′ | 93 |
| HCV-2a/c | 5′ HEX- GAG TAC ACC IGA ATT GCC GGG 3′ | 151 |
| HCV-2b | 5′ FAM- TGA GTA CAC CGG AAT TMC CG 3′ | 152 |
| β-actin-F | ACCGAGCGCGGCTACAG | 60 |
| β-actin-R | CTTAATGTCACGCACGATTTCC | |
| β-actin-probe | FAM-TTCACCACCACGGCCGAGC-BHQ1 |
Abbreviation: HCV, hepatitis C virus.
3.8. Statistical Analysis
Frequency and percentage were used for categorizing qualitative variables. All data were analyzed using the statistical package for the social sciences (SPSS), version 22 (IBM), and GraphPad Prism, version 8.0.2 (Graphpad Software, Inc.). The P value < 0.05 was considered statistically significant.
4. Results
We enrolled 100 hemodialysis patients with an average age of 60.3 ± 13.8, including 61 men with an average age of 58.1 ± 14.9 and 39 women with an average age of 63.6 ± 11.4. Most patients were married (77%), without university education (86%), without a governmental career (74%), and living in urban areas (86%), which in the intra-group comparison of each parameter, a significant relationship was observed (Table 2). Also, CKD was the primary cause of hemodialysis, and DM and/or hypertension were the leading causes of CKD in this study (Table 3). Additionally, most patients (78%) had been undergoing hemodialysis for at least two years, 89% had hemodialysis three times per week (3 × WHD), and 93% had dialysis for at least 3.5 hours or more per session.
| Parameters | No. (%) | P-Value | HCV+ (%) | HCV- (%) | P-Value |
|---|---|---|---|---|---|
| Age | < 0.0001 | 0.126 | |||
| ≤ 50 | 20 (20) | 2 | 18 | ||
| > 50 | 80 (80) | 2 | 78 | ||
| Sex | 0.028 | 0.558 | |||
| Male | 61 (61) | 3 | 58 | ||
| Female | 39 (39) | 1 | 38 | ||
| Marital status | < 0.0001 | NA | |||
| Married | 77 (77) | 2 | 75 | ||
| Single | 8 (8) | 0 | 8 | ||
| Others | 15 (15) | 2 | 13 | ||
| Education | < 0.0001 | NA | |||
| High school | 62 (62) | 4 | 58 | ||
| Diploma | 24 (24) | 0 | 24 | ||
| Bachelor and higher | 14 (14) | 0 | 14 | ||
| Occupation | < 0.0001 | NA | |||
| Self-employment | 74 (74) | 4 | 70 | ||
| Government | 26 (26) | 0 | 26 | ||
| Location | < 0.0001 | NA | |||
| Urban | 86 (86) | 4 | 82 | ||
| Rural | 14 (14) | 0 | 14 |
Abbreviation: HCV, hepatitis C virus.
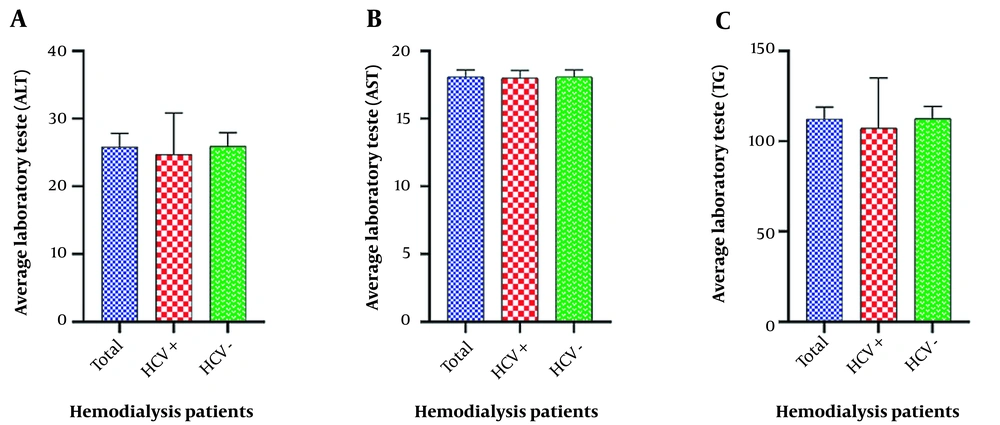
Based on patient records, less than half of the patients (49%) had a history of blood transfusion, of whom 43 had only one blood transfusion. Thalassemia status was not reported in any of the patients. Opium use was reported in 11% of the patients, and only one patient had a history of imprisonment (1%). No history of microbial or viral infections (except viral hepatitis) was recorded in any of the patients (Table 3). Laboratory tests were in the normal range, including alanine transaminase (ALT) 18.4 ± 25.8 U/L, aspartate aminotransferase (AST) 18.1 ± 4.6 U/L, and triglycerides (TG) 112.3 ± 65.4 mg/dL. Although about 20% of the patients had an abnormal TG level (up to 150 mg/dL), the average level of AST and ALT were in the normal range (Figure 1).
| Parameters | No. (%) | P-Value | HCV+ | HCV- | P-Value |
|---|---|---|---|---|---|
| Blood transfusion history | 0.841 | 0.618 | |||
| No | 51 (51) | 3 | 48 | ||
| 1 ≤ | 49 (49) | 1 | 48 | ||
| Thalassemia | NA | NA | |||
| Yes | 0 | 0 | 0 | ||
| No | 100 (100) | 4 | 96 | ||
| History of drug use | < 0.0001 | 0.337 | |||
| Yes | 11 (11) | 1 | 10 | ||
| No | 89 (89) | 3 | 86 | ||
| Cause of dialysis | < 0.0001 | NA | |||
| Diabetes | 31 (31) | 1 | 30 | ||
| Hypertension | 13 (13) | 0 | 13 | ||
| Diabetes and hypertension | 27 (27) | 2 | 25 | ||
| Polycystic | 8 (8) | 1 | 7 | ||
| Kidney atrophy | 3 (3) | 0 | 3 | ||
| Others | 18 (18) | 0 | 18 |
Abbreviation: HCV, hepatitis C virus.
The serological and molecular tests demonstrated the occurrence of anti-HCV antibodies in hemodialysis patients at a prevalence of 3% compared to a 1% prevalence of OCI. Although no significant correlation was observed between HCV and gender, education, and occupation, most HCV-positive cases were observed in men, patients with low education levels, and freelance occupation (Table 2). The genotyping results of HCV-positive cases indicated a higher prevalence of genotype 1a in the patient serum samples. On the other hand, genotype 3a was observed in the PBMC samples that indicated OCI.
5. Discussion
The prevalence of anti-HCV antibody and OCI among these patients was 3% and 1%, respectively. In this regard, Kargar Kheirabad et al. studied the prevalence of HCV among hemodialysis patients in Hormozgan, southern Iran. They reported a rate of 3.36%, comparable to the current study's findings (18). The lowest prevalence of OCI in hemodialysis patients was reported by Eslamifar et al. and Taherpour et al., who found prevalence rates of 0% and 1.6%, respectively (19, 20). Compared to the study of Zahedi et al., in 2012, on the seroprevalence of HBV, HIV, and HCV among hemodialysis patients in Kerman, the prevalence of HCV among these patients decreased by 4% in this study (21). This decrease could be attributed to various factors, including more accurate screening of patients in the dialysis ward for the presence of antigens or antibodies of infectious agents, screening of blood products in the blood transfusion organization for antigens or antibodies of infectious agents, use of separate dialysis machines for patients infected with infectious agents, routine sterilization and disinfection of devices and wards, and general standards of training and proficiency, which can play an important role in reducing the transmission and spread of infectious agents among patients.
In addition, Ashkani-Esfahani et al., in a meta-analysis in 2017, investigated the seroprevalence of HCV among hemodialysis patients in Middle East countries. Egypt and Syria, with a prevalence of more than 50%, and Iran and Lebanon, with a prevalence of less than 13%, were reported as countries with the highest and lowest prevalence rates, respectively (22). On the other hand, Dolatimehr et al., in a systematic review in 2017, reported the prevalence of OCI among hemodialysis patients in different countries from 0% in Iran to 45% in Spain (14). Similar to previous studies, the most common genotype among HCV-positive (serology) patients was 1a, while genotype 3a was observed in only one case. In this line, in a meta-analysis by Mahmud et al., genotypes 1 and 3 were reported as the most common genotypes among the Iranian population, with a frequency of 56% and 39%, respectively (23). In addition, in another study by Mahmoudvand et al., genotype 3a was reported as the most prevalent genotype among patients with OCI, and a significant association was found between this genotype and dialysis patients (9).
Based on our findings, DM and hypertension, the leading causes of CKD, have contributed to the increased number of patients referred to dialysis centers, similar to the results reported by other studies (24). This study identified old age, low education level, self-employment, and urban living risk factors for CKD. Therefore, adequate knowledge of CKD risk factors and their management can enhance the conditions and complications of patients and dialysis wards. In a study conducted in the Persian Gulf countries, the prevalence of DM was reported from 45 - 74% among hemodialysis patients (25). Also, the prevalence of hypertension in different studies has been reported as 50 - 60%, even up to 85% among hemodialysis patients (15).
As in numerous previous studies, men accounted for a greater proportion of dialysis patients in this study. In Hecking's study, which investigated 35,964 sampled dialysis outcomes and practice patterns, the prevalence of men undergoing dialysis compared to women was reported as 59% vs. 41% across all age groups. Additionally, women were found to be treated with dialysis to a lesser extent than men (26). The difference in sex hormones and their receptors between men and women is possibly one of the most important reasons for more male dialysis patients. It has been reported that male sex hormones, such as testosterone, play a role in increasing oxidative stress, stimulating the RAS, and increasing fibrosis in the damaged kidney, worsening the disease.
On the other hand, female sex hormones such as estrogen have a protective effect on fibrosis, inflammation, oxidative stress, and inhibition of the RAS (27). In this study, contrary to the vast majority of previous research on HCV infection, the prevalence of HCV (anti-HCV and OCI) was not statistically correlated with the male gender. In other studies, men were more susceptible to HCV infection due to some factors, including the protective effect of female hormones (17-oestradiol and estrogen) during reproductive age, the greater likelihood of spontaneous clearance of the virus in the female sex, and men's high-risk behavior, including sexual relations with men (MSM) and sharing infected needles (28-30).
Age over 60 years, one of the risk factors for kidney disease in many studies, was not an exception in this study, and the average age of the patients was 60.3 ± 13.8 (31). In addition, similar to previous studies, men developed kidney disease and required dialysis treatment younger and earlier than women (32). Unhealthy lifestyles, women's more attention to self-care, the destructive effects of testosterone in men, and the protective effects of estrogen in women can be considered the most important reasons in this part of the study (32). Other parameters such as education level, place of residence, and occupation were also influential factors among dialysis patients in this study. Park et al.'s study in 2021 indicated an inverse relationship between the education level and the rate of CKD (33). The results of the current study are also in line with this finding, with 5% of patients having a secondary degree. These results show the need to pay attention to educating the people in society. As higher education can be associated with improved socio-economic status, deprivation index, number of family members, and higher income, it can largely prevent risk factors such as DM, hypertension, obesity, and harmful behaviors such as smoking (33).
Self-employment, which had the largest share among the patients in this study, had a direct relationship with education level. Among 40 patients who had a freelance job, more than half of them (25%) had a bachelor's degree, while out of 26 patients who had government jobs, only 8 had the secondary degree. Therefore, like education level, the type of job also has an inverse relationship with the rate of CKD and the need for dialysis. Similar to this finding, Adjei et al.'s study in 2017, the HELIUS study, utilizing a multi-ethnic sample, reported the relationship between low-level jobs and worse kidney outcomes among all ethnic groups (34). This study found that individuals in low-level jobs are more likely to be exposed to biological substances or nephrotoxins such as lead, mercury, organic solvents, welding gases, glycol ethers, and grain dust. Additionally, these individuals may have an unhealthy lifestyle and diet, which can contribute to developing various diseases, including heart and chronic kidney diseases (34). The current study, which was conducted in a middle-income country, similar to the results of Jagannathan and Patzer's study, showed that CKD and the need for dialysis were more common among urban people than rural people (35).
5.1. Conclusions
Briefly, the current study demonstrated that several factors and conditions, such as lifestyle, occupation, educational level, and disinfection of dialysis wards and machines, play an important role in managing and controlling the risk factors of hemodialysis patients, including HCV and OCI.