1. Background
Acinetobacter baumannii is an opportunistic pathogen associated with serious infections, such as septicemia, endocarditis, pneumonia, meningitis, and wound infections. It poses a major public health problem as it can resist harsh environmental conditions and various antibiotics. Available antibiotic options for multidrug-resistant (MDR) A. baumannii infections are limited because of increasing resistance and lack of new antibiotics (1). Various treatment regimens such as colistin-, sulbactam-, and tigecycline-based antimicrobial treatments have been reported; however, it is difficult to find optimized treatment strategies for MDR A. baumannii infections (2, 3).
Colistin is one of the last therapeutic options for MDR A. baumannii infections and has been used as a rescue therapy for severe infections. However, colistin resistance has recently been reported worldwide (4). According to a multicenter study in Korea, colistin-carbapenem combination therapy and sulbactam-containing regimens considerably decreased the mortality rate of patients infected with carbapenem-resistant A. baumannii (CRAB) (3). Although controversial, combination therapy is considered superior to monotherapy because it often improves clinical outcomes by increasing antibiotic efficacy and decreasing the probability of resistance development (1, 5).
Many studies have investigated the synergistic effects of combinations of various antibiotics and antimicrobial peptides (6-9). Antibacterial peptides have a broad antibacterial spectrum owing to various antibacterial mechanisms; in particular, there is no or low resistance in bacteria owing to their unique microbicidal activity that targets the plasma membrane and causes cell death after membrane destruction (10, 11). Antimicrobial peptides have recently received widespread attention as a promising class of compounds that can be used in combination with classical antibiotics to treat various infections (7, 12).
Short synthetic cationic lipopeptides exhibit excellent broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria (13). Among these peptides, synthetic short proline-rich lipopeptides (SPRLPs) are amphiphilic cationic peptides with potent Gram-negative antibacterial activities that act via membrane rupture and lysis (8). Among the SPRLPs, C12-PRP can potentiate the antimicrobial activity of minocycline and rifampin against drug-resistant gram-negative pathogens, particularly Pseudomonas aeruginosa. Although C12-PRP with L-amino acids has a better adjuvant potency than C12-prp with D-amino acids in combination with rifampin against MDR/extensively drug-resistant (XDR) P. aeruginosa, C12-PRP is susceptible to non-specific proteolytic degradation by human proteases (8). This indicates that the in vivo efficacy of C12-PRP was lower than that of C12-prp.
Mastoparans are positively charged peptides extracted from wasp venom that are 14 amino acids in length and rich in hydrophobic and basic amino acids (14). Mastoparan-AF exhibits excellent antibacterial activity against various pathogens, including Staphylococcus aureus, Klebsiella pneumoniae, P. aeruginosa, and multiple antibiotic-resistant Escherichia coli O157:H7 (15, 16). Mastoparan-loaded chitosan nanoconstructs showed synergistic bactericidal effects against MDR A. baumannii; hence, they could be used as potential therapeutic agents (17).
2. Objectives
To date, there have only been a few reports on the efficacy of the combination therapy with amphiphilic peptides C12-prp or mastoparan and various antibiotics against drug-resistant A. baumannii clinical isolates. In this study, we investigated the synergistic effect and bactericidal activity of a combination of antibiotics and peptides (C12-prp, mastoparan) against A. baumannii clinical isolates consisting of 24 XDR and 11 pan-drug-resistant (PDR) strains.
3. Methods
3.1. Bacterial Strains and Drugs
We used 35 A. baumannii clinical isolates collected from January 2004 to December 2014 at Chosun University Hospital, Gwangju, Korea, which consisted of 24 XDR and 11 PDR strains. Acinetobacter baumannii was initially identified using the VITEK 2 system (bioMérieux, Marcy d'Etoile, France). Thereafter, species identification was performed via blaOXA-51-like and gyrB multiplex polymerase chain reaction (18, 19). Antimicrobial sensitivity testing was performed using the broth microdilution method according to the Sensititre DKMGN panel (Thermo Fisher Scientific, East Grinstead, UK) and VITEK 2 automated system (bioMérieux, France).
Staphylococcus aureus (ATCC 29213) and Pseudomonas aeruginosa (ATCC 27853) were used as quality controls. Antibiotics were divided into nine categories according to the criteria presented by Magiorakos et al. XDR strains were defined to be resistant to at least one agent in all but two or fewer antimicrobial categories, while PDR strains were defined to be resistant to all agents in all antimicrobial categories (20). Two peptides, C12-prp (CH3(CH2)10CO-prprprp-NH2 [all D1-peptide]) and mastoparan (mastoparan-AF; INLKALAALAKKIL-NH2), used in this study were synthesized by ANYGEN Co., Ltd. (Gwangju, Korea) (8, 9). All synthetic peptides had > 95% purity. The lyophilized powder was dissolved in sterile distilled water and stored at -70°C until further use. Antibiotics (ceftazidime, cefepime, ciprofloxacin, colistin, meropenem, and rifampicin) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Susceptibility Testing of C12-prp and Mastoparan
The minimum inhibitory concentrations (MIC) of C12-prp and mastoparan were determined using the broth dilution method, as described by the Clinical and Laboratory Standards Institute (21). Various concentrations of C12-prp and mastoparan, ranging from 128-0.0625 μg/mL, were used for MIC determination. Briefly, each peptide was added to the Muller-Hinton (MH) broth (BD, USA) with a bacterial inoculum of 5 × 105 colony-forming unit (CFU)/mL and incubated at 37°C for 18 to 20 h. After incubation, the MIC was recorded as the lowest concentration of the agent that showed no visible bacterial growth.
3.3. Multiple Combination Bactericidal Test
We investigated antibiotic-peptide combinations that inhibited the growth of A. baumannii clinical isolates via multiple combination bactericidal test (MCBT) (22). The following peptides and antibiotics were used individually and in combination: 32 µg/mL C12-prp, 2 µg/mL mastoparan, 2 µg/mL ceftazidime, 2 µg/mL cefepime, 2 µg/mL ciprofloxacin, 2 µg/mL colistin, 2 µg/mL meropenem, and 2 µg/mL rifampicin. Antibiotic and peptide solutions were prepared at a concentration 10 times higher than that to be added for each test. Multiple combination bactericidal tests were performed in a 96-well plate (Corning, Amsterdam, the Netherlands). Briefly, approximately 5 × 105 CFU/mL of bacteria were inoculated in MH broth containing antibiotics and peptides individually or in combinations (200 μL volume per well of a 96 well-plate) and incubated for 48 h at 37°C. After incubation, 10 μL of cell contents with no visible turbidity and 10 μL of 10-fold diluted suspension were plated directly onto MH agar plates (BD, USA) and incubated for 24 h at 37°C. Antibiotic-peptide combinations with no colony growth were identified and used in further studies. All experiments were performed in triplicate.
3.4. Time-Kill Assay
Two antibiotics (colistin and rifampicin) that were most effective in inhibiting the growth of A. baumannii clinical isolates in combination with each peptide were selected via MCBT and used for time-kill assay with four combinations (C12-prp-colistin, C12-prp-rifampicin, mastoparan-colistin, and mastoparan-rifampicin). The same concentrations of antibiotics and peptides as those used for MCBT were used in the time-kill study. Each agent was added to 10 mL of MH broth (BD, USA) with a bacterial inoculum of approximately 1 × 106 CFU/mL and incubated in a shaking incubator at 37°C for 24 h. Colony enumeration with 10-fold serially diluted suspensions was performed at 0- and 24-h time points.
Bactericidal activity was defined as a ≥ 3 log10 CFU/mL reduction compared to the initial inoculum at 24 h. As different researchers tend to use different criteria for judging synergism, two commonly used criteria (criteria 1 and 2) were applied in this study. Synergy criterion 1 was defined as ≥ 2 log10 CFU/mL reduction with the combination compared to the most active single agent at 24 h. Synergy criterion 2 was defined as ≥ 2 log10 CFU/mL reduction with the combination compared with the most active single agent at 24 h and ≥ 2 log10 CFU/mL reduction below the initial inoculum at 24 h. Antagonism was defined as a ≥ 2 log10 CFU/mL increase with the combination compared to the most active single agent at 24 h. Indifference was defined as a < 2 log10 change in CFU/mL with the combination compared to the most active single agent at 24 h (23, 24).
4. Results
4.1. Determination of the Minimum Inhibitory Concentrations of C12-prp and Mastoparan and the Result of Multiple Combination Bactericidal Testing in Acinetobacter baumannii Clinical Isolates
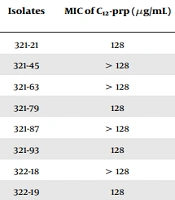
We determined the MIC for 35 A. baumannii clinical isolates, and the MIC range was 128 to > 128 µg/mL for C12-prp and 2 - 16 µg/mL for mastoparan (Table 1). For the MCBT of antibiotics and peptides, we used 32 µg/mL of C12-prp and 2 µg/mL of mastoparan, which is 1/4 of the MIC50 obtained from 35 A. baumannii clinical isolates. MIC50 is the MIC required to inhibit the growth of 50% of the clinical isolates. The antibiotic-peptide combinations that inhibited the growth of A. baumannii clinical isolates were investigated using the MCBT assay (Table 2).
When C12-prp, mastoparan, ceftazidime, cefepime, ciprofloxacin, colistin, meropenem, and rifampicin were used alone, no bactericidal activity was observed against any of the 35 clinical isolates (data not shown). Among the 12 combinations, the C12-prp-colistin, C12-prp-rifampicin, mastoparan-colistin, and mastoparan-rifampicin combinations showed more than 94.3% (33/35) inhibitory activity against the XDR and PDR strains of A. baumannii clinical isolates. However, the combinations of C12-prp or mastoparan peptide with ceftazidime, cefepime, ciprofloxacin, and meropenem did not inhibit the bacterial growth of any of the 35 clinical isolates.
| Isolates | MIC of C12-prp (µg/mL) | MIC of Mastoparan (µg/mL) | |
|---|---|---|---|
| XDR | 321-21 | 128 | 4 |
| XDR | 321-45 | > 128 | 4 |
| XDR | 321-63 | > 128 | 8 |
| XDR | 321-79 | 128 | 4 |
| XDR | 321-87 | > 128 | 8 |
| XDR | 321-93 | 128 | 4 |
| XDR | 322-18 | > 128 | 8 |
| XDR | 322-19 | 128 | 16 |
| XDR | 322-23 | 128 | 16 |
| XDR | 322-45 | 128 | 4 |
| XDR | 322-49 | > 128 | 8 |
| XDR | 322-94 | 128 | 16 |
| XDR | 323-21 | 128 | 8 |
| XDR | 323-52 | > 128 | 4 |
| XDR | 323-74 | 128 | 8 |
| XDR | 324-45 | > 128 | 8 |
| XDR | 324-77 | 128 | 8 |
| XDR | 325-51 | > 128 | 4 |
| XDR | 325-74 | > 128 | 8 |
| XDR | 326-77 | 128 | 8 |
| XDR | 326-79 | > 128 | 8 |
| XDR | 327-62 | 128 | 8 |
| XDR | 327-69 | > 128 | 4 |
| XDR | 327-75 | > 128 | 2 |
| PDR | 348-11 | 128 | 8 |
| PDR | 348-12 | > 128 | 8 |
| PDR | 348-13 | > 128 | 16 |
| PDR | 348-14 | > 128 | 16 |
| PDR | 348-15 | 128 | 8 |
| PDR | 348-22 | > 128 | 16 |
| PDR | 348-23 | 128 | 16 |
| PDR | 348-24 | 128 | 16 |
| PDR | 348-25 | > 128 | 16 |
| PDR | 348-26 | > 128 | 16 |
| PDR | 348-33 | > 128 | 8 |
Determination of Minimum Inhibitory Concentration of C12-prp and Mastoparan in Acinetobacter baumannii Clinical Isolates
| Combination of Antibiotics and Peptides a | % (No.) of Acinetobacter baumannii Clinical Isolates Inhibited |
|---|---|
| C12-prp-ceftazdime | 0 (0/35) |
| C12-prp-cepefime | 0 (0/35) |
| C12-prp-ciprofloxacin | 0 (0/35) |
| C12-prp-colistin | 97.1 (34/35) |
| C12-prp-meropenem | 0 (0/35) |
| C12-prp-rifampicin | 100 (35/35) |
| Mastoparan-ceftazdime | 0 (0/35) |
| Mastoparan-cepefime | 0 (0/35) |
| Mastoparan-ciprofloxacin | 0 (0/35) |
| Mastoparan-colistin | 94.3 (33/35) |
| Mastoparan-meropenem | 0 (0/35) |
| Mastoparan-rifampicin | 94.3 (33/35) |
Results of Multiple Combination Bactericidal Tests According to the Combination of Amphiphilic Peptides (C12-prp, Mastoparan) and Classical Antibiotics Against Extensively Drug-Resistant and Pandrug-Resistant Groups of Acinetobacter baumannii Clinical Isolates
4.2. Results of the Time-Kill Assays
We performed time-kill assays for the C12-prp-colistin, C12-prp-rifampicin, mastoparan-colistin, and mastoparan-rifampicin combinations in 35 A. baumannii clinical isolates. When 32 µg/mL of C12-crp or 2 µg/mL of colistin were added alone, bactericidal activity with a reduction of ≥ 3 log10 CFU/mL compared with the initial inoculums at 24 h was not observed in any of the 35 clinical isolates. However, in the C12-prp-colistin combination group, bactericidal activity was observed in 22 out of 24 XDR strains but not in the 11 PDR strains. Synergy on applying criterion 1 was observed in all 24 (100%) XDR strains and in one out of 11 (9.1%) PDR strains in the C12-prp-colistin combination. Synergy on applying criterion 2 was observed in 23 out of 24 (95.8%) XDR strains but not in the PDR strains. In addition, no antagonism was observed for any of the 35 clinical isolates (Tables 3 and 4).
| Bacterial Strain | Log10 (VC) at 24 h | Log10 (VC of Combination) - Log10 (VCMASA) at 24 h | Synergy | Log10 (VC of 24 h) - Log10 (VC of 0 h) | Synergy | BA e | Log10 (VC) at 24 h | Log10 (VC of combination) - Log10 (VCMASA) at 24 h | Synergy | Log10 (VC of 24 h) - Log10 (VC of 0 h) | Synergy | BA e | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C12-prp a | Colistin b | C12-prp + Colistin | Criteria 1 c | C12-prp + Colistin | Criteria 2 d | C12-prp + Colistin | C12-prp a | Rifampicin b | C12-prp + Rifampicin | Criteria 1 c | C12-prp + Rifampicin | Criteria 2 d | C12-prp + Rifampicin | |||
| 321-21 | 10.88 | 8.32 | 0.00 | -8.32 | S | -6.69 | S | B | 10.88 | 7.81 | 0.00 | -7.81 | S | -6.69 | S | B |
| 321-45 | 10.84 | 9.10 | 0.00 | -9.10 | S | -6.87 | S | B | 10.84 | 7.51 | 0.00 | -7.51 | S | -6.87 | S | B |
| 321-63 | 10.71 | 7.82 | 0.00 | -7.82 | S | -5.97 | S | B | 10.71 | 7.93 | 0.00 | -7.93 | S | -5.97 | S | B |
| 321-79 | 10.65 | 7.60 | 0.00 | -7.60 | S | -5.91 | S | B | 10.65 | 7.73 | 0.00 | -7.73 | S | -5.91 | S | B |
| 321-87 | 10.82 | 7.90 | 3.12 | -4.78 | S | -2.76 | S | NB | 10.82 | 7.61 | 0.00 | -7.61 | S | -5.88 | S | B |
| 321-93 | 10.86 | 7.67 | 0.00 | -7.67 | S | -5.92 | S | B | 10.86 | 7.77 | 0.00 | -7.77 | S | -5.92 | S | B |
| 322-18 | 10.88 | 8.23 | 3.56 | -4.67 | S | -3.17 | S | B | 10.88 | 7.20 | 2.00 | -5.20 | S | -4.74 | S | B |
| 322-19 | 10.64 | 8.17 | 0.00 | -8.17 | S | -6.79 | S | B | 10.64 | 6.80 | 0.00 | -6.80 | S | -6.79 | S | B |
| 322-23 | 10.67 | 7.71 | 0.00 | -7.71 | S | -5.59 | S | B | 10.67 | 7.96 | 0.00 | -7.96 | S | -5.59 | S | B |
| 322-45 | 10.44 | 7.85 | 0.00 | -7.85 | S | -5.44 | S | B | 10.44 | 7.82 | 3.30 | -4.52 | S | -2.14 | S | NB |
| 322-49 | 10.84 | 7.81 | 0.00 | -7.81 | S | -5.65 | S | B | 10.84 | 6.82 | 0.00 | -6.82 | S | -5.65 | S | B |
| 322-94 | 10.88 | 7.74 | 3.88 | -3.85 | S | -1.77 | I | NB | 10.88 | 7.86 | 0.00 | -7.86 | S | -5.66 | S | B |
| 323-21 | 10.80 | 7.52 | 0.00 | -7.52 | S | -6.85 | S | B | 10.80 | 6.73 | 0.00 | -6.73 | S | -6.85 | S | B |
| 323-52 | 10.94 | 9.23 | 0.00 | -9.23 | S | -6.66 | S | B | 10.94 | 8.08 | 0.00 | -8.08 | S | -6.66 | S | B |
| 323-74 | 10.57 | 8.08 | 0.00 | -8.08 | S | -6.37 | S | B | 10.57 | 7.61 | 3.67 | -3.94 | S | -2.70 | S | NB |
| 324-45 | 10.93 | 8.82 | 0.00 | -8.82 | S | -5.71 | S | B | 10.93 | 7.95 | 0.00 | -7.95 | S | -5.71 | S | B |
| 324-77 | 11.01 | 7.72 | 0.00 | -7.72 | S | -5.59 | S | B | 11.01 | 8.38 | 0.00 | -8.38 | S | -5.59 | S | B |
| 325-51 | 10.90 | 7.95 | 0.00 | -7.95 | S | -5.75 | S | B | 10.90 | 8.02 | 0.00 | -8.02 | S | -5.75 | S | B |
| 325-74 | 10.91 | 7.72 | 0.00 | -7.72 | S | -5.92 | S | B | 10.91 | 7.56 | 0.00 | -7.56 | S | -5.92 | S | B |
| 326-77 | 10.79 | 7.62 | 0.00 | -7.62 | S | -5.95 | S | B | 10.79 | 7.63 | 0.00 | -7.63 | S | -5.95 | S | B |
| 326-79 | 10.71 | 8.58 | 0.00 | -8.58 | S | -5.91 | S | B | 10.71 | 7.77 | 0.00 | -7.77 | S | -5.91 | S | B |
| 327-62 | 10.76 | 7.72 | 0.00 | -7.72 | S | -5.90 | S | B | 10.76 | 7.53 | 0.00 | -7.53 | S | -5.90 | S | B |
| 327-69 | 10.74 | 7.53 | 0.00 | -7.53 | S | -5.93 | S | B | 10.74 | 7.47 | 0.00 | -7.47 | S | -5.93 | S | B |
| 327-75 | 10.78 | 7.41 | 0.00 | -7.41 | S | -5.67 | S | B | 10.78 | 7.90 | 0.00 | -7.90 | S | -5.67 | S | B |
| 348-11 | 10.95 | 8.83 | 5.98 | -2.85 | S | -0.65 | I | NB | 10.95 | 7.89 | 6.96 | -0.93 | I | 0.32 | I | NB |
| 348-12 | 11.01 | 7.97 | 6.90 | -1.08 | I | 1.02 | I | NB | 11.01 | 7.99 | 6.99 | -1.01 | I | 1.11 | I | NB |
| 348-13 | 10.97 | 7.95 | 6.95 | -1.00 | I | 0.96 | I | NB | 10.97 | 7.18 | 5.92 | -1.25 | I | -0.06 | I | NB |
| 348-14 | 10.90 | 7.96 | 6.92 | -1.04 | I | 1.00 | I | NB | 10.90 | 6.93 | 6.80 | -0.13 | I | 0.89 | I | NB |
| 348-15 | 10.88 | 6.83 | 6.84 | 0.01 | I | 0.94 | I | NB | 10.88 | 6.92 | 6.80 | -0.13 | I | 0.89 | I | NB |
| 348-22 | 10.89 | 7.88 | 6.99 | -0.89 | I | 0.19 | I | NB | 10.89 | 6.90 | 6.99 | 0.09 | I | 0.19 | I | NB |
| 348-23 | 10.95 | 7.92 | 6.93 | -0.99 | I | 0.12 | I | NB | 10.95 | 7.92 | 6.86 | -1.07 | I | 0.04 | I | NB |
| 348-24 | 10.81 | 7.90 | 6.75 | -1.14 | I | -0.19 | I | NB | 10.81 | 6.77 | 6.81 | 0.04 | I | -0.13 | I | NB |
| 348-25 | 10.94 | 6.93 | 6.85 | -0.08 | I | 0.05 | I | NB | 10.94 | 7.86 | 6.91 | -0.95 | I | 0.11 | I | NB |
| 348-26 | 10.74 | 7.79 | 7.92 | 0.12 | I | 2.10 | I | NB | 10.74 | 7.88 | 7.97 | 0.09 | I | 2.15 | I | NB |
| 348-33 | 10.84 | 6.90 | 5.91 | -0.99 | I | 0.05 | I | NB | 10.84 | 6.84 | 6.77 | -0.07 | I | 0.90 | I | NB |
Results of Time-Kill Assay and Bactericidality of C12-prp-Colistin and C12-prp-Rifampicin Combinations Against Acinetobacter baumannii Clinical Isolates
| Combination of Antibiotics and Peptides and Interaction | XDR % (No.) | PDR % (No.) | Total % (No.) |
|---|---|---|---|
| C12-prp + colistin | |||
| Synergy (criteria 1) | 100 (24/24) | 9.1 (1/11) | 71.4 (25/35) |
| Synergy (criteria 2) | 95.8 (23/24) | 0 (0/11) | 65.7 (23/35) |
| Indifference | 4.2 (1/24) | 100 (11/11) | 34.3 (12/35) |
| Antagonism | 0 (0/24) | 0 (0/11) | 0 (0/35) |
| Bactericidality | 91.7 (22/24) | 0 (0/11) | 62.9 (22/35) |
| C12-prp + rifampicin | |||
| Synergy (criteria 1) | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
| Synergy (criteria 2) | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
| Indifference | 0 (0/24) | 100 (11/11) | 31.4 (11/35) |
| Antagonism | 0 (0/24) | 0 (0/11) | 0 (0/35) |
| Bactericidality | 91.7 (22/24) | 0 (0/11) | 62.9 (22/35) |
| Mastoparan + colistin | |||
| Synergy (criteria 1) | 100 (24/24) | 9.1 (1/11) | 71.4 (25/35) |
| Synergy (criteria 2) | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
| Indifference | 0 (0/24) | 100 (11/11) | 31.4 (11/35) |
| Antagonism | 0 (0/24) | 0 (0/11) | 0 (0/35) |
| Bactericidality | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
| Mastoparan + rifampicin | |||
| Synergy (criteria 1) | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
| Synergy (criteria 2) | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
| Indifference | 0 (0/24) | 100 (11/11) | 31.4 (11/35) |
| Antagonism | 0 (0/24) | 0 (0/11) | 0 (0/35) |
| Bactericidality | 100 (24/24) | 0 (0/11) | 68.6 (24/35) |
Comparison of Time-Kill Assay Results of Peptides (C12-prp, Mastoparan) and Antibiotics Combination Against Extensively Drug-Resistant and Pandrug-Resistant Groups of Acinetobacter baumannii Clinical Isolates
In the C12-prp-rifampicin combination assay, the addition of 2 µg/mL rifampicin alone did not significantly inhibit the bacterial growth, but the combination of C12-prp-rifampicin showed bactericidal activity in 22 out of 24 (91.7%) XDR strains, but not in the PDR strains. In addition, synergy on applying criteria 1 and 2 was observed in all 24 (100%) XDR strains but not in the PDR strains, and antagonism was not observed in any of the 35 clinical isolates with the C12-prp-rifampicin combination (Tables 3 and 4). In the mastoparan-colistin combination assay, bactericidal activity was not observed in any of the 35 clinical isolates in the mastoparan or colistin-alone groups. However, with the mastoparan-colistin combination, bactericidal activity was observed in all 24 (100%) XDR strains but not in the 11 PDR strains. Synergy on applying criterion 1 was observed in all 24 (100%) XDR strains and in one out of 11 (9.1%) PDR strains in the mastoparan-colistin combination. Synergy on applying criterion 2 was observed in all 24 (100%) XDR strains but not in PDR strains. In addition, no antagonism was observed in any of the 35 clinical isolates (Tables 5 and 4).
| Bacterial Strain | Log10 (VC) at 24 h | Log10 (VC of combination) - Log10 (VCMASA) at 24 h | Synergy | Log10 (VC of 24 h) - Log10 (VC of 0 h) | Synergy | BA e | Log10 (VC) at 24 h | Log10 (VC of Combination) - Log10 (VCMASA) at 24 h | Synergy | Log10 (VC of 24 h) - Log10 (VC of 0 h) | Synergy | BA e | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS a | Colistin b | MS + Colistin | Criteria 1 c | MS + Colistin | Criteria 2 d | MS + Colistin | MS a | Rifampicin b | MS + Rifampicin | Criteria 1 c | MS + Rifampicin | Criteria 2 d | MS + Rifampicin | |||
| 321-21 | 10.92 | 7.95 | 0.00 | -7.95 | S | -5.95 | S | B | 10.92 | 7.03 | 0.00 | -7.03 | S | -5.95 | S | B |
| 321-45 | 10.88 | 6.79 | 0.00 | -6.79 | S | -6.00 | S | B | 10.88 | 7.97 | 0.00 | -7.97 | S | -6.00 | S | B |
| 321-63 | 10.69 | 6.88 | 0.00 | -6.88 | S | -6.96 | S | B | 10.69 | 6.91 | 0.00 | -6.91 | S | -6.96 | S | B |
| 321-79 | 10.91 | 6.90 | 0.00 | -6.90 | S | -6.93 | S | B | 10.91 | 6.92 | 0.00 | -6.92 | S | -6.93 | S | B |
| 321-87 | 10.91 | 6.94 | 0.00 | -6.94 | S | -6.90 | S | B | 10.91 | 6.83 | 0.00 | -6.83 | S | -6.90 | S | B |
| 321-93 | 10.87 | 7.83 | 0.00 | -7.83 | S | -5.71 | S | B | 10.87 | 7.95 | 0.00 | -7.95 | S | -5.71 | S | B |
| 322-18 | 10.95 | 7.01 | 0.00 | -7.01 | S | -5.81 | S | B | 10.95 | 6.40 | 0.00 | -6.40 | S | -5.81 | S | B |
| 322-19 | 10.72 | 6.72 | 0.00 | -6.72 | S | -6.90 | S | B | 10.72 | 6.76 | 0.00 | -6.76 | S | -6.90 | S | B |
| 322-23 | 10.92 | 6.95 | 0.00 | -6.95 | S | -6.72 | S | B | 10.92 | 6.88 | 0.00 | -6.88 | S | -6.72 | S | B |
| 322-45 | 10.94 | 6.83 | 0.00 | -6.83 | S | -6.91 | S | B | 10.94 | 6.62 | 0.00 | -6.62 | S | -6.91 | S | B |
| 322-49 | 10.81 | 6.90 | 0.00 | -6.90 | S | -6.88 | S | B | 10.81 | 6.78 | 0.00 | -6.78 | S | -6.88 | S | B |
| 322-94 | 10.87 | 7.78 | 0.00 | -7.78 | S | -5.90 | S | B | 10.87 | 6.80 | 0.00 | -6.80 | S | -5.90 | S | B |
| 323-21 | 10.96 | 6.91 | 0.00 | -6.91 | S | -5.96 | S | B | 10.96 | 6.86 | 0.00 | -6.86 | S | -5.96 | S | B |
| 323-52 | 10.92 | 6.79 | 0.00 | -6.79 | S | -5.97 | S | B | 10.92 | 6.83 | 0.00 | -6.83 | S | -5.97 | S | B |
| 323-74 | 10.91 | 6.75 | 0.00 | -6.75 | S | -5.90 | S | B | 10.91 | 6.94 | 0.00 | -6.94 | S | -5.90 | S | B |
| 324-45 | 10.64 | 7.93 | 0.00 | -7.93 | S | -5.72 | S | B | 10.64 | 7.93 | 0.00 | -7.93 | S | -5.72 | S | B |
| 324-77 | 10.86 | 6.71 | 0.00 | -6.71 | S | -5.66 | S | B | 10.86 | 6.04 | 0.00 | -6.04 | S | -5.66 | S | B |
| 325-51 | 10.61 | 6.91 | 0.00 | -6.91 | S | -5.88 | S | B | 10.61 | 6.90 | 0.00 | -6.90 | S | -5.88 | S | B |
| 325-74 | 10.77 | 6.97 | 0.00 | -6.97 | S | -5.70 | S | B | 10.77 | 6.88 | 0.00 | -6.88 | S | -5.70 | S | B |
| 326-77 | 10.70 | 6.93 | 0.00 | -6.93 | S | -5.80 | S | B | 10.70 | 6.93 | 0.00 | -6.93 | S | -5.80 | S | B |
| 326-79 | 10.69 | 6.93 | 0.00 | -6.93 | S | -5.68 | S | B | 10.69 | 6.89 | 0.00 | -6.89 | S | -5.68 | S | B |
| 327-62 | 10.86 | 6.76 | 0.00 | -6.76 | S | -5.84 | S | B | 10.86 | 6.78 | 0.00 | -6.78 | S | -5.84 | S | B |
| 327-69 | 10.76 | 6.95 | 0.00 | -6.95 | S | -5.85 | S | B | 10.76 | 6.95 | 0.00 | -6.95 | S | -5.85 | S | B |
| 327-75 | 10.91 | 6.88 | 0.00 | -6.88 | S | -5.94 | S | B | 10.91 | 6.86 | 0.00 | -6.86 | S | -5.94 | S | B |
| 348-11 | 10.94 | 8.98 | 5.90 | -3.07 | S | -0.84 | I | NB | 10.94 | 7.99 | 6.90 | -1.10 | I | 0.16 | I | NB |
| 348-12 | 10.92 | 8.02 | 6.90 | -1.12 | I | 1.03 | I | NB | 10.92 | 7.91 | 7.00 | -0.91 | I | 1.13 | I | NB |
| 348-13 | 11.03 | 7.95 | 7.04 | -0.91 | I | 1.05 | I | NB | 11.03 | 7.19 | 5.97 | -1.23 | I | -0.02 | I | NB |
| 348-14 | 11.03 | 7.89 | 6.00 | -1.89 | I | 0.03 | I | NB | 11.03 | 6.96 | 6.92 | -0.04 | I | 0.95 | I | NB |
| 348-15 | 10.89 | 6.79 | 7.01 | 0.23 | I | 1.08 | I | NB | 10.89 | 7.02 | 7.00 | -0.02 | I | 1.06 | I | NB |
| 348-22 | 10.84 | 7.94 | 6.99 | -0.94 | I | 0.11 | I | NB | 10.84 | 6.92 | 7.00 | 0.08 | I | 0.12 | I | NB |
| 348-23 | 10.96 | 7.90 | 7.06 | -0.84 | I | 0.27 | I | NB | 10.96 | 7.79 | 6.95 | -0.84 | I | 0.16 | I | NB |
| 348-24 | 10.91 | 7.95 | 7.07 | -0.88 | I | 0.07 | I | NB | 10.91 | 6.85 | 6.98 | 0.14 | I | -0.02 | I | NB |
| 348-25 | 11.02 | 6.95 | 6.98 | 0.04 | I | 0.04 | I | NB | 11.02 | 7.85 | 6.90 | -0.95 | I | -0.04 | I | NB |
| 348-26 | 10.91 | 7.99 | 7.97 | -0.01 | I | 1.95 | I | NB | 10.91 | 7.95 | 7.93 | -0.02 | I | 1.90 | I | NB |
| 348-33 | 10.90 | 6.78 | 6.00 | -0.78 | I | 0.23 | I | NB | 10.90 | 6.79 | 7.09 | 0.30 | I | 1.33 | I | NB |
Results of Time-Kill Assay and Bactericidality of Mastoparan-Colistin and Mastoparan-Rifampicin Combinations Against Acinetobacter baumannii Clinical Isolates
In the mastoparan-rifampicin combination assay, the addition of 2 µg/mL of mastoparan or 2 µg/mL rifampicin alone did not significantly inhibit the bacterial growth, but the combination of mastoparan-rifampicin showed bactericidal activity in all 24 (100%) XDR strains, but not in the 11 PDR strains. In addition, synergy on applying criteria 1 and 2 was observed in all 24 (100%) XDR strains but not in the PDR strains, and antagonism was not seen in any of the 35 clinical isolates with the mastoparan-rifampicin combination (Tables 5 and 4).
5. Discussion
Combination therapy using various drugs against Acinetobacter infections remains controversial. However, several in vitro studies have reported the synergistic effects of combination therapy for treating Acinetobacter infections and have postulated it to be beneficial despite a lack of direct evidence (25). Antibiotic resistance of A. baumannii has been continuously reported, increasing the need for new therapies to overcome this resistance. In particular, treating Acinetobacter infections caused by the MDR, XDR, and PDR groups of A. baumannii strains is a real medical challenge. This study demonstrated that C12-prp and mastoparan peptides exhibit potent synergistic antibacterial activity when combined with colistin or rifampicin against XDR A. baumannii clinical isolates.
The combination of C12-prp-colistin showed 100% (24/24) synergy against XDR A. baumannii strains; however, only 9.1% (1/11) against PDR strains. It is believed that the amphiphilic cationic lipopeptide C12-prp and cationic polypeptide colistin synergistically destroy the outer membrane of XDR A. baumannii and exhibit bactericidal activity. Domalaon et al. reported that SPRLP and C12-PRP could potentiate the efficacy of minocycline and rifampin by disrupting the bacterial membrane, followed by enhanced antibiotic uptake (8).
Proteins and naturally occurring peptides are composed of amino acids in L-configuration. Therefore, proteins composed of D-amino acids have high resistance to protease degradation and low immunogenicity in vivo compared to those composed of L-amino acids, which increase their gut, blood, and intracellular half-life (26). Therefore, in this study, we chose the D-lipopeptide analog C12-prp, rather than C12-PRP with L-amino acids, for the antibiotic-antimicrobial peptide combination study. Domalaon et al. reported that amphiphilic C12-PRP might be susceptible to non-specific proteolysis by human proteases, whereas proteolysis-resistant C12-prp retains its adjuvant properties with a slightly decreased potency compared to C12-PRP (8).
The potential of combination therapy with colistin, lipopeptide, or glycopeptide antibiotics for treating MDR A. baumannii has been reported (27). Gordon et al. reported combining colistin and the glycopeptide antibiotic vancomycin showed synergistic effects and sustained bactericidal activity against multidrug-resistant A. baumannii strains. The results of the E-test for 34 MDR A. baumannii clinical isolates showed that vancomycin MIC was reduced from > 256 μg/mL to ≤ 48 μg/mL with 0.5 μg/mL colistin. In addition, a combination of 20 μg/mL vancomycin and 1 μg/mL colistin showed bactericidal activity, except for the regrowth of one strain in a time-kill study using ATCC 19606 and five colistin-susceptible MDR A. baumannii strains (28).
Galani et al. reported synergy in 16 (53.3%) of 30 combinations tested with various concentrations of colistin (0.25 ×, 0.5 ×, and 1 × MIC) and 10 μg/mL of the lipopeptide antibiotic daptomycin in time-kill studies using 10 colistin-susceptible MDR A. baumannii isolates (29). The cationic polypeptide colistin interacts with the anionic lipopolysaccharide layer of gram-negative bacteria and induces the osmotic lysis of cells. According to Gordon et al. (28) as a synergistic mechanism of colistin-vancomycin combination, the cell-permeabilizing properties of colistin disrupt the outer membrane of the bacteria, thereby improving the penetration of vancomycin through the A. baumannii outer membrane to reach the action site of the cell wall (27, 28).
In this study, mastoparan-colistin and mastoparan-rifampicin combinations showed 100% (24/24) synergy against XDR strains in the time-kill assay. However, in the case of PDR A. baumannii strains, only mastoparan-colistin showed 9.1% (1/11) synergy, whereas the mastoparan-rifampicin combination showed no synergistic effect. Vila-Farres et al. reported that mastoparan isolated from the Vespula lewisii venom exhibited good antimicrobial activity with lower MICs against colistin-susceptible and colistin-resistant A. baumannii than other antimicrobial peptides such as buforin I, β-defensin (30).
In a 2017 study by Lin et al., the combined effects of mastoparan-AF and clinically used antibiotics were evaluated against seven MDR A. baumannii clinical isolates using the fractional inhibitory concentration index. Mastoparan-AF showed synergistic activity against six of the seven MDR A. baumannii strains when combined with colistin, two strains when combined with ciprofloxacin, and six strains when combined with trimethoprim/sulfamethoxazole. However, mastoparan-AF showed no difference when combined with ampicillin, cephalothin, gentamicin, or neomycin (9). In 2023, Lin et al. reported that mastoparan-AF killed multiple antibiotic-resistant hemolytic E. coli O157:H7 cells through multiple membrane disruption patterns by adopting the 3 - 11 amphipathic helix-type structure of mastoparan, facilitating membrane interaction (15).
Therefore, it is important to identify the appropriate combination of antibiotics for combination therapy in treating infectious diseases. The synergy rates of antibiotics or antibiotic-antimicrobial peptide combinations may vary according to the test method used, such as the time-kill assay, E-test, Checkerboard test, type of antibiotics, and degree of bacterial resistance (31). Aaron et al. reported that MCBT can be a useful technique to screen bactericidal antibiotic combinations for treating cystic fibrosis associated with Burkholderia cepacia strains (22). In this study, we used the MCBT assay to identify antibiotic-peptide combinations that inhibited the growth of A. baumannii clinical isolates and found that four combinations (C12-prp-colistin, C12-prp-rifampicin, mastoparan-colistin, and mastoparan-rifampicin) presented more than 94.3% inhibitory activity against XDR and PDR A. baumannii clinical isolates. When used alone, the peptides and antibiotics at the concentrations used in MCBT did not show any inhibitory effects.
Antimicrobial peptides may serve as an alternative treatment option for bacterial infections. Antimicrobial peptides have a broad antibiotic spectrum and are not affected by classical resistance mechanisms to conventional antibiotics; therefore, they are frequently used to develop new therapeutic strategies (32). As the development of new therapeutic agents to treat multidrug-resistant gram-negative bacterial infections, especially A. baumannii infections, is urgently required, antimicrobial peptides that show strong synergistic effects when combined with classical antibiotics can be excellent alternatives for developing new therapeutic strategies. In this study, the potential of C12-prp-colistin and mastoparan-rifampicin combinations to inhibit multidrug-resistant A. baumannii growth was reported for the first time.
5.1. Conclusions
In conclusion, C12-prp and mastoparan showed 100% synergy and ≥ 91.7% bactericidal activity (91.7% for C12-prp and 100% for mastoparan) in combination with rifampicin or colistin against 24 XDR A. baumannii clinical isolates. However, for the 11 PDR A. baumannii clinical isolates, only two combinations, C12-prp-colistin and mastoparan-colistin, showed 9.1% (1/11) synergy. These results indicate that C12-prp and mastoparan peptides show potent synergistic antibacterial activity when combined with colistin or rifampicin, making them good candidates for the in vivo studies of XDR A. baumannii infections.