1. Introduction
Fulminant myocarditis is the most serious and unique type of myocarditis, characterized by sudden onset, rapid progression, rapid occurrence of hemodynamic abnormalities and severe arrhythmias, and multiple organ failure (1). Pseudomonas aeruginosa is a gram-negative bacterium widely distributed in nature and healthcare facilities. In hospitals, P. aeruginosa is often spread as an opportunistic pathogen to cause hospital-acquired infections (2). The infections are difficult to treat due to the complex mechanism of drug resistance (3). This study analyzed the anti-infection treatment process and pharmaceutical care of a patient with P. aeruginosa infection secondary to fulminant myocarditis.
2. Case Presentation
A previously healthy 28-year-old woman weighing 55 kg was admitted to emergency intensive care unit (EICU) on January 12, 2022, with a dry cough for 3 days, subxiphoid pain for 1 day, and dampness and cold limbs for 2 hours. Three days before admission, the patient developed an itchy throat, accompanied by a dry cough, sore throat, nasal congestion, runny nose, fear of cold, and fever. The laboratory examination results were as follows: White blood cell (WBC) count, 7.58 × 109/L; neutrophil ratio (N%), 64.3%; platelet count, 129 × 109/L; C-reactive protein (CRP), 1.5 mg/L; cytokine (CK), 457 U/L; CK-MB, 29 U/L; cardiac troponin I (cTnI), 2.170 μg/L; N-terminal forebrain natriuretic peptide (NTpro-BNP), 6510 ng/L; and creatinine (Cr), 66.9 μmol/L.
After emergency treatment with piperacillin sodium tazobactam sodium 4.5 g q8h, esomeprazole sodium 40 mg bid, and intermittent rehydration, the subxiphoid pain worsened than before. Two hours later, the patient became agitated, cold, and sweaty, and the blood pressure suddenly dropped to 56/30 mmHg. Therefore, norepinephrine was given to increase blood pressure. One hour later, the patient’s blood pressure dropped again, and her limbs became wetter and colder. The patient was immediately transferred to the EICU for further treatment. On admission, the patient was delirious and unresponsive. The temperature was 34.5°C, the heart rate was 140 beats per minute, and the breaths were 10 times per minute. The patient was diagnosed with fulminant myocarditis, shock, acute heart failure, and metabolic acidosis.
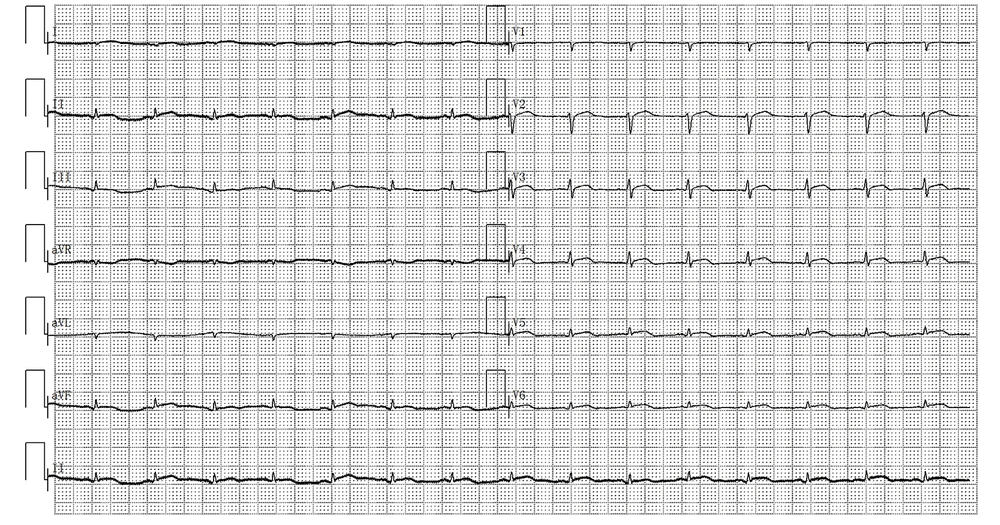
At the EICU, the patient was given fasting, gastrointestinal decompression, emergency, and tracheal intubation respirator-assisted ventilation. Then, ECMO and CRRT were applied with other supportive measures. The routine blood examination showed a WBC of 21.56 × 109/L, N% of 93.6%, CRP of 85.7 mg/L, and procalcitonin (PCT) of 5.82 ng/mL. During days 1 to 6 after admission, the patient’s body temperature was maintained at about 36.5°C. The beta-D-glucan test and bacterial culture were both negative. On day 2 of hospitalization, the ECG showed ST elevation, low voltage, and right axis deviation (Figure 1). On the sixth day after admission, the 24h urine volume of the patient was only 70 mL. The WBC count was 20.24 × 109/L, N% was 88.7%, CRP was 161 mg/L, and PCT was 3.21 ng/mL.
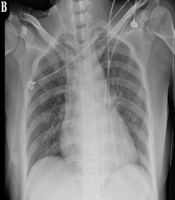
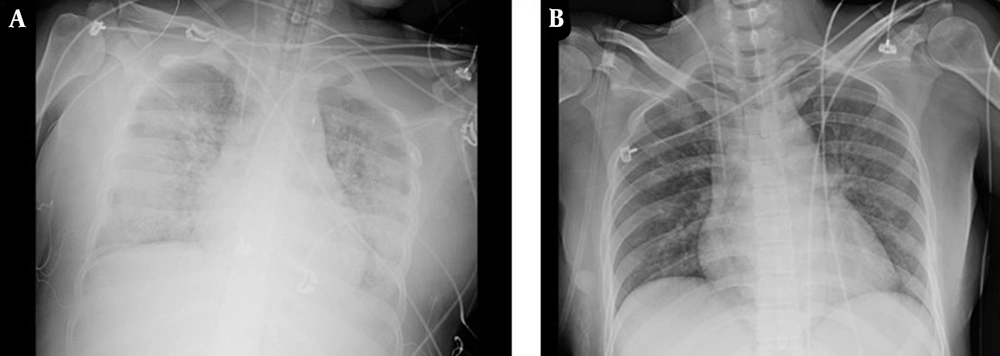
The chest radiograph showed exudative changes in both lungs and aggravation of pulmonary edema (Figure 2A). On the seventh day after admission, the patient had a large amount of white sputum in the airway. Her sputum culture showed the growth of P. aeruginosa, and a drug sensitivity test showed that the organism was sensitive to amikacin and gentamicin (Table 1). Clinical pharmacists recommended piperacillin sodium tazobactam (4.5 g q8h) (4 hours of infusion) combined with amikacin (400 mg qd) for anti-infection. On the 10th day after admission, a large amount of yellow mucous sputum appeared in the airway.
| Pseudomonas aeruginosa+++ | ||
|---|---|---|
| Antibiotics | MIC (μg/mL) | Results |
| Imipenem | ≥ 16 | Resistant |
| Ampicillin | ≥ 32 | Resistant |
| Ampicillin/Sulbactam | ≥ 32 | Resistant |
| Cefazolin | ≥ 64 | Resistant |
| Ceftazidime | 16 | Intermediary |
| Cefepime | 16 | Intermediary |
| Cefotetan | ≥ 64 | Resistant |
| Amikacin | 4 | Sensitive |
| Gentamicin | 4 | Sensitive |
| Ciprofloxacin | ≥ 4 | Resistant |
| Levofloxacin | ≥ 8 | Resistant |
| SMZco | ≥ 320 | Resistant |
| Piperacillin/Tazobactam | 64 | Intermediary |
| Tobramycin | ≤ 1 | Sensitive |
Drug Sensitivity Test of Pseudomonas aeruginosa
Laboratory tests showed a WBC count of 32.36 × 109/L and N% of 95.6%. The levels of CRP and PCT were 182.2 mg/L and 2.73 ng/mL, respectively. Bronchoalveolar lavage fluid (BALF) and blood samples were collected for metagenomics Next-generation Sequencing (mNGS). The mNGS results were both positive for P. aeruginosa. On the 13th day after admission, the patient’s cardiac function gradually recovered, and ECMO was stopped. She was anuric, and CRRT continued. The drug sensitivity results showed that P. aeruginosa was sensitive to ceftazidime-avibactam and amikacin. Therefore, the anti-infective regimen was adjusted to ceftazidime-avibactam 2.5 g q8h (maintenance infusion over 2 hours) combined with amikacin 0.4 g qd. On the 13th to 16th days after admission, the patient’s highest temperature was 39°C, and there was a small amount of thin white sputum in the airway. Laboratory tests showed a WBC count of 16.99 × 109/L, N% of 83.6%, CRP level of 69.8 mg/L, and PCT level of 0.82 g/mL. On the 23rd day after admission, the patient’s highest temperature was 38.4°C, and laboratory tests showed a WBC count of 13.7 × 109/L. The levels of CRP and PCT were 28.4 mg/L and 1.79 ng/mL, respectively.
The 24h urine volume was 5 mL, and the patient had intermittent mental symptoms. The clinical pharmacologist believed that the psychiatric symptoms might be related to ceftazidime-avibactam, and the poor renal function recovery might be related to amikacin. Therefore, it was suggested that the dose of ceftazidime-avibactam should be adjusted to 1.25 g q8h, and amikacin should be stopped. The patient showed no obvious psychiatric symptoms again. On the 27th day after admission, the patient stopped CRRT. On the 29th day after admission, the patient’s body temperature was 38.4°C. Laboratory tests showed a WBC count of 8.69 × 109/L, N% of 73.3%, CRP level of 39.5 mg/L, and PCT level of 1.29 g/mL. The chest X-ray showed significant improvement in pneumonia (Figure 2B).
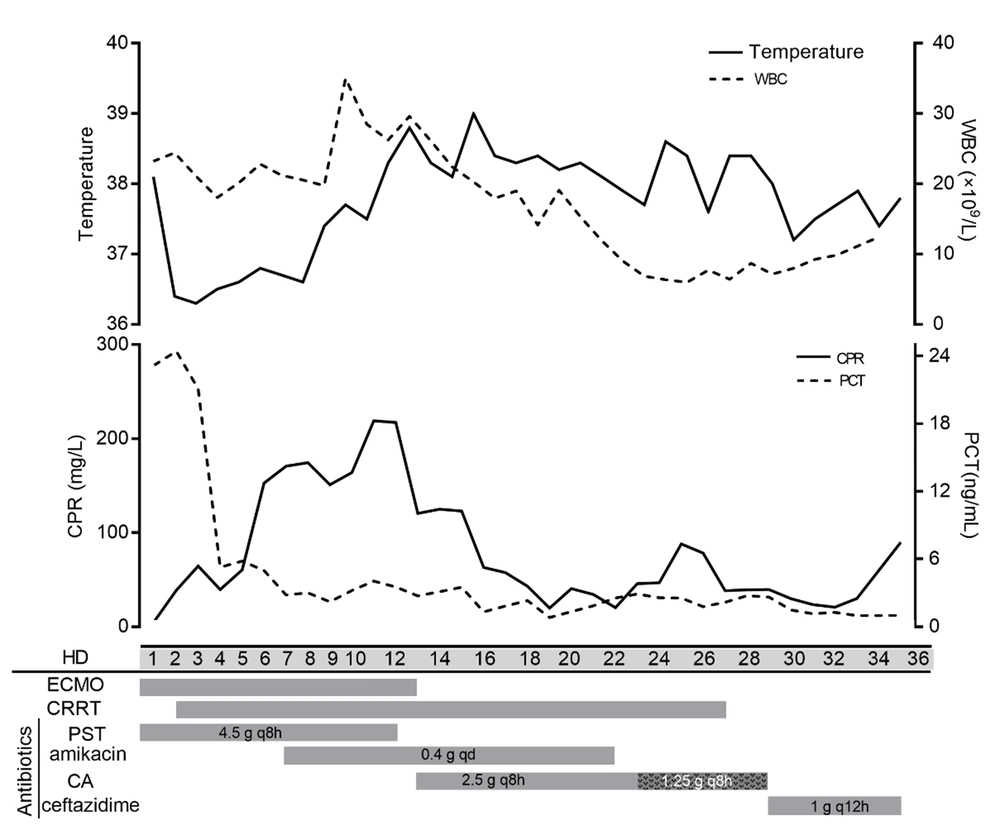
Blood culture was negative, whereas sputum culture showed the growth of Stenotrophomonas maltophilia, which was sensitive to ceftazidime. The 24h urine output was 105 mL. Thus, ceftazidime-avibactam was replaced with ceftazidime (1 g q12h) for anti-infection. Thirty-five days after admission, the patient had a 24h urine volume of 1700 mL, Cr of 187 μmol/L, and blood urea nitrogen of 26.2 mmol/L, and she was discharged after renal function improved. The clinical course of the patient’s laboratory findings during admission is demonstrated in Figure 3.
3. Discussion
In this case, the young woman had no previous systemic disease. The onset of this disease was sudden, with the precursor symptoms of upper respiratory tract virus infection and the rapid emergence of severe hemodynamic disorders. The laboratory test showed that the myocardial zymogram and troponin were significantly increased, indicating that the myocardium was seriously damaged. The bedside echocardiography showed that the diffuse ventricular wall movement was weakened. After admission, P. aeruginosa was detected, and mNGS confirmed its sequence. The three aspects could be used to determine whether P. aeruginosa is a pathogenic bacterium. First, the patient received ECMO combined with CRRT support therapy, indwelling internal jugular vein, femoral vein catheters, intubation, and ventilator-assisted ventilation.
The skin and mucosal barrier were destroyed, and methylprednisolone sodium succinate regulated immunotherapy for 15 days. Therefore, there were risk factors for P. aeruginosa infection. Second, mNGS can detect a variety of pathogenic microorganisms without bias by sequencing and analyzing microbial and host nucleic acids in clinical samples (4). Huang et al. showed that mNGS had higher accuracy and sensitivity (5). The patient’s mNGS results showed that P. aeruginosa was detected in both BALF and blood samples. Three consecutive sputum cultures showed P. aeruginosa, consistent with the mNGS results. Finally, according to the combined drug sensitivity results, the treatment protocol was adjusted to ceftazidime-avibactam combined with amikacin. After about 2 weeks of treatment, the patient’s inflammatory indicators returned to normal. No P. aeruginosa was detected again.
The drug sensitivity results showed that P. aeruginosa was not sensitive to cephalosporins, carbapenems, quinolones, and common β-lactamase inhibitor compound preparations, which could be considered multidrug-resistant P. aeruginosa (6). The combination regimen for the treatment of multidrug-resistant P. aeruginosa infections was recommended by Tamma et al., and the β-lactam combined with aminoglycosides, quinolones, or fosfomycin was most commonly used against P. aeruginosa (7). Considering drug sensitivity results, clinical pharmacists suggested piperacillin sodium tazobactam combined with amikacin for anti-infection therapy. During CRRT treatment, drugs with water solubility, low protein binding (PB) rate, small distribution volume (Vd), small relative molecular weight, and mainly metabolized by the kidney are easy to be cleared by CRRT, so the dosage needs to be adjusted (8). During ECMO support therapy, the PK/PD of antibiotics often changed, mainly because ECMO could increase the Vd of hydrophilic drugs and ECMO circuit adsorption of lipophilic and high PB drugs resulting in a decrease in the concentration of antibiotics. Therefore, it was recommended to increase the loading dose during ECMO for hydrophilic drugs with small Vd (8).
Piperacillin sodium tazobactam and amikacin are water-soluble drugs, mainly metabolized by the kidney, with PB of 16 - 48% and 0 - 10% and Vd of 0.24/0.4 L/kg and 0.26 L/kg, respectively. Dose adjustment may be necessary under ECMO combined with CRRT support therapy. However, Donadello et al.’s study (9) showed no significant difference in the serum concentration and PK parameters of piperacillin sodium tazobactam between ECMO and non-ECMO patients. Also, Gelisse et al.’s study (10) showed that ECMO had no significant effect on the peak and trough plasma concentrations of amikacin. The amikacin dose of 7.5 mg/kg q24h for patients undergoing CRRT and 4.5 g q8h for piperacillin sodium tazobactam against P. aeruginosa (16 mg/L < minimum drug concentration (MIC) ≤ 64 mg/L) was recommended by Gilbert et al. (11). Therefore, the patient was initially given piperacillin sodium tazobactam 4.5 g q8h combined with amikacin 0.4 g qd anti-infective therapy.
After 5 days of anti-infection treatment, the patient’s body temperature was higher than before, and there was still a large amount of yellow mucus in the airway. The following reasons might exist for the poor anti-infection efficacy: First, under ECMO combined with CRRT treatment, Vd significantly increased, leading to a decrease in plasma concentration, which could not reach the expected PK/PD target value. Second, the drug sensitivity results showed that the MIC of piperacillin sodium tazobactam against P. aeruginosa was 64 mg/L (intermediate sensitivity). Although piperacillin sodium tazobactam was given in full dose and extended infusion time, the decrease in blood concentration may lead to sub-inhibitory concentration.
Studies have shown that low inhibitory concentrations could induce resistance of P. aeruginosa to long-term exposure to antibiotics and even induce clinically sensitive P. aeruginosa to be resistant strains (12). On the 13th day after admission, the drug sensitivity results showed that P. aeruginosa was sensitive to ceftazidime-avibactam and amikacin. Ceftazidime had strong anti-P. aeruginosa activity, and its MIC against ceftazidime was 16 μg/mL (mediator). As a new β-lactamase inhibitor, avibactam itself does not have antibacterial activity, but it can restore and strengthen the activity of ceftazidime against P. aeruginosa (13). In addition, ceftazidime-avibactam has a good clinical effect on infection caused by multidrug-resistant P. aeruginosa (14).
The recommended dose of ceftazidime-avibactam under CRRT is 1.25 g q8h, according to Gilbert et al. (11). Soukup et al.’s study (15) showed that patients with P. aeruginosa pneumonia treated with CRRT needed a higher dose (2.5 g q8h) of ceftazidime-avibactam when 4 μg/mL ≤ MIC ≤ 8 μg/mL. The anti-infective therapy was adjusted to ceftazidime-avibactam 2.5 g q8h combined with amikacin 0.4 g qd. On the 19th day after admission, the patient developed delirium, panic, and other psychiatric symptoms. Clinical pharmacists believed that the psychiatric symptoms might be an adverse reaction to the large dose of ceftazidime-avibactam. The dose of ceftazidime-avibactam was suggested to be reduced to 1.25 g q8h, after which the patient did not appear delirium or panic. Finally, the infection index of the patient returned to normal. Chest X-ray showed significant improvement in pulmonary infection. Therefore, the anti-infection treatment was effective.
3.1. Conclusions
We presented a case of fulminant myocarditis secondary to multidrug-resistant P. aeruginosa infection. Clinical pharmacists actively assisted the doctors in developing an anti-infection regimen. Initially, piperacillin sodium tazobactam combined with amikacin was used for anti-infection therapy, which had a poor clinical effect. Subsequently, combined with the drug sensitivity test and relevant guidelines, it was recommended to use ceftazidime-avibactam and amikacin for anti-infection. Meantime, adverse reactions were found in time to adjust the dose to ensure the safety of medication for patients. In clinical practice, selecting correct and effective drugs according to etiology is necessary, considering the influence of patient life support on antimicrobial PK/PD. However, more randomized studies are still needed to demonstrate the efficacy and safety of ceftazidime-avibactam and amikacin for multidrug-resistant P. aeruginosa infection patients during ECMO and CRRT therapy.