1. Background
Antibiotic-associated diarrhea is caused by Clostridium difficile, a type of spore-forming bacillus that is anaerobic and gram-positive. Clostridium difficile infection (CDI) is a critical nosocomial illness that poses a significant threat to patient health, with high rates of morbidity and potential mortality (1). Over the past two decades, the incidence and severity of CDI have risen dramatically in Europe and North America, thereby imposing a substantial financial burden on healthcare systems worldwide (2). Clostridium difficile infection is projected to incur a financial burden of approximately 1.9 to 7 billion US dollars per year on the United States healthcare system. This infection contributes to an average extension of hospital stays by 2.8 to 10.4 days (3).
The emergence of hypervirulent variants of toxigenic C. difficile (BI/NAP1/027) in 2003 has led to a rapid spread of this epidemic strain throughout Europe and North America (4, 5). However, C. difficile 027 infections are not commonly reported in Asia. A recent meta-analysis showed that although CDI rates were similar across Asia, Europe, and North America, the hypervirulent ribotype 027 was much less commonly detected in Asia, with a prevalence of only 0.3% (6). Several factors that increase the risk of the NAP1/027 strain have been identified, including advanced age, previous use of proton-pump inhibitors (PPIs), and prior CDI within the past 12 months (7-9). Previous studies have investigated the clinical outcomes of NAP1/027 patients; nevertheless, whether this strain can predict CDI severity, recurrence, or increased mortality is still controversial (10).
NAP1/027 infections have only been reported sporadically in China, with cases documented in Beijing, Zhejiang, Hong Kong, and Shandong (11-14). However, the detection of C. difficile in China might be hindered by a low index of clinical suspicion and the limited availability of laboratory testing.
2. Objectives
This study aimed to investigate the clinical characteristics of NAP1/027 strains and identify their associated risk factors. The primary objective was to gain a deeper insight into the clinical features, risk factors, and outcomes of NAP1/027 infection. The findings of this study can contribute to the early prevention and control of nosocomial infections caused by this particular strain, thereby improving healthcare practices and patient outcomes.
3. Methods
This retrospective case-control study was conducted at a university-affiliated teaching hospital (Shandong Provincial Hospital affiliated to Shandong First Medical University), with a bed capacity of 3000 located in Shandong, China. This study retrospectively reviewed the medical records of admitted patients aged 18 years or older with a positive C. difficile polymerase chain reaction (PCR) assessed by Cepheid Xpert C. difficile assay from June 2018 to August 2021. The stool sample of each patient was classified as negative or positive for C. difficile and the NAP1/027 strain. Diarrhea was referred to as having > 3 unformed stools within a 24-hour period, based on the Bristol Stool Chart types 5 - 7 (15). The diagnosis of CDI was made by considering both laboratory results and clinical manifestations, which included the clinical evidence of pseudomembranous colitis or the presence of diarrhea and a stool test positive for the Cepheid Xpert C. difficile assay (16). For each CDI case caused by the NAP1/027 strain, three control patients with non-NAP1/027 CDI were selected.
The selection of controls was based on age and use of medical services to ensure a comparable group. Hospital-onset CDI was referred to as a positive result for CDI PCR that occurred 48 hours after hospitalization or within 12 weeks after discharge from a healthcare facility. For each patient, only one stool sample was collected. The exclusion criteria for patients, regardless of the strain they tested positive for, included age under 18 years, previous CDI diagnosis, or outpatient diagnosis for their initial CDI infection. The following markers were used to assess the severity of CDI:
Elevated white blood cell count (WBC >15000 cells/mL), serum creatinine (CRE) levels 1.5 times higher than the patient’s baseline, toxic megacolon, ileus, fever (> 38°C), low serum albumin levels (< 2.5 g/dL), and colitis findings on computed tomography scans, according to the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America 2010 criteria (16).
The clinical data of the enrolled patients, including demographic information such as age, gender, whether they were in a healthcare-associated or community-associated setting, and their background diseases, were recorded. The study also documented potential risk factors in the month before diarrhea onset, such as broad-spectrum antibiotic exposure (defined as carbapenems, cephalosporins, clindamycin, piperacillin-tazobactam, fluoroquinolones, or combination therapy), PPIs, immunosuppressive agents, chemotherapy, abdominal surgery, nasogastric intubation, and hospitalization 90 days before diagnosis. This study also recorded biological parameters, including the percentage of neutrophil granulocytes, WBC, blood platelet count, hemoglobin, glutamate aspartate transaminase (AST), albumin, fecal occult blood, C-reactive protein (CRP), and serum CRE. Clinical manifestations, such as fever, abdominal pain, hematochezia, and vomiting, were also documented. All laboratory results collected within 3 days of CDI diagnosis were also recorded.
The results are expressed as frequency and percentage for categorical variables. However, mean and standard deviation (normal distribution) or median and quartile (non-normal distribution) express continuous variables. To assess the differences in clinical data between NAP1/027 patients and non-NAP1/027 controls, data distribution was first examined using the D’Agostino-Pearson test. For continuous data that followed a normal distribution, the Student’s t-test was applied. However, if the data did not meet the normality assumption, the Mann-Whitney U test was employed. Meanwhile, categorical data were analyzed using the chi-square (χ2) test. To identify the potential predictors of the NAP1 strain, an exploratory univariate logistic regression model was constructed. Only significant variables (P < 0.05) in the univariable analysis were included in the model. The logistic regression data are presented as odds ratio (OR) and 95% confidence interval (CI). All the statistical tests were conducted with SPSS software (version 16.0). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in prior approval by the Shandong Provincial Hospital affiliated to Shandong First Medical University’s human research committee.
4. Results
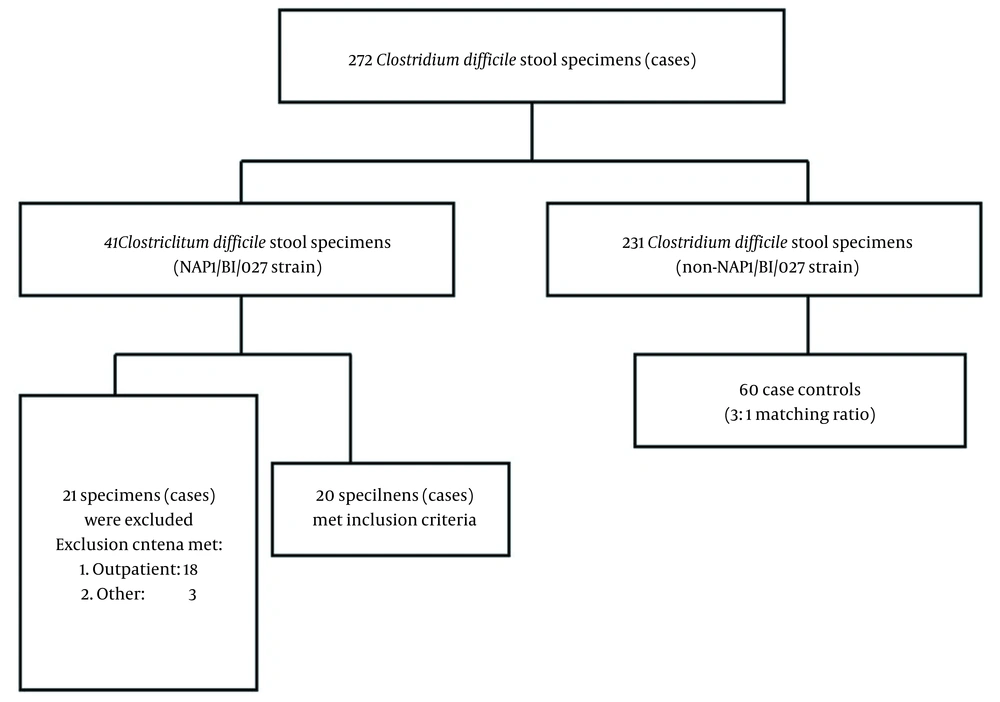
This study was carried out during a non-epidemic CDI time period. Throughout the study duration, the Cepheid Xpert C. difficile test identified 272 stool specimens that were positive for C. difficile. Among these specimens, 41 cases (15.07%) tested positive for NAP1/027. Out of these 41 cases, 20 met the inclusion criteria (Figure 1). The patients with NAP1/027 CDI had significantly increased odds of recent hospitalization within the 90 days prior to their CDI diagnosis when compared to controls infected with a non-NAP1 strain (90% vs. 62%, P = 0.002). The patients with NAP1/027 CDI also had a higher median CRP level (69.50 vs. 16.25, P = 0.01) and a higher usage of third- and fourth-generation cephalosporins (15% vs. 8%, P = 0.043). However, the cases with the NAP1/027 strain had a lower median AST level (11.5 vs. 19.5, P = 0.00) and less cortisol drug usage, defined as the use of prednisolone-equivalent dosage > 10 mg/day within 1 month, compared to non-NAP1/027 controls.
There was no significant difference in the median levels of serum CRE between the NAP1 and non-NAP1 groups, with values of 66.2 and 71, respectively (P = 0.356). Likewise, no remarkable difference was observed in the median levels of WBC, which were 8.4 and 6.93 in the NAP1 and non-NAP1 groups, respectively (P = 0.1) (Table 1). There was also no difference in the number of cases with WBC counts > 15 (× 109/L) or serum CRE levels > 195 μmol/L between the two groups. Additionally, there were no cases of ileus or shock during CDI diagnosis in either the NAP1 or non-NAP1 groups.
| Variables | NAP1 (N = 20) | Non-NAP1 (N = 60) | P-Value |
|---|---|---|---|
| Patient demographics | |||
| Median age (IQR) | 70 (58 - 79) | 66 (59 - 77) | 0.772 |
| Male gender | 11 (0.55) | 35 (0.58) | 0.897 |
| Hospitalized in the last 90 days | 18 (0.9) | 37 (0.62) | 0.002 |
| Medical history | |||
| Cardiovascular disease | 7 (0.35) | 31 (0.52) | 0.227 |
| Any malignancy | 3 (0.15) | 13 (0.22) | 0.223 |
| Diabetes mellitus | 6 (0.3) | 13 (0.22) | 0.66 |
| Chronic kidney disease | 3 (0.15) | 9 (0.15) | 0.861 |
| Hypertension | 11 (0.55) | 35 (0.58) | 0.897 |
| Recent surgery within 1 month | 5 (0.25) | 11 (0.18) | 0.636 |
| Pulmonary disease | 5 (0.25) | 12 (0.2) | 0.883 |
| Chemotherapy | 1 (0.05) | 8 (0.13) | 0.307 |
| Gastrointestinal surgery | 1 (0.05) | 4 (0.07) | 0.624 |
| Markers of CDI severity | |||
| Peak WBC at diagnosis | 9.0625 | 9.2623 | |
| Abdominal tenderness at diagnosis | 7 (0.35) | 15 (0.25) | 0.783 |
| Presence of shock at diagnosis | 0 | 0 | |
| Presence of ileus at diagnosis | 0 | 0 | |
| Biological parameters | |||
| Median WBC (IQR) | 8.4 (6.38 - 16.3) | 6.93 (5.33 - 10.88) | 0.1 |
| WBC count > 15 × 109/L | 6 (0.3) | 8 (0.13) | 0.137 |
| NEU% (IQR) | 77.85 (63.62 - 87.97) | 71.8 (65.25 - 80.75) | 0.203 |
| Hb (g/L) | 98.25 ± 4.74 | 105.1 ± 3.058 | 0.254 |
| PLT (× 109/L) | 241.15 ± 23.87 | 227.3 ± 14.63 | 0.633 |
| ALB (g/L) | 31.11 ± 1.45 | 32.90 ± 0.83 | 0.275 |
| Hypoalbuminemia (< 35 g/L) | 13 (0.77) | 17 (0.28) | 0.076 |
| AST (U/L) (IQR) | 11.5 (6.25 - 16.25) | 19.5 (11 - 31) | 0.000 |
| CRE (μmol/L) (IQR) | 66.2 (48.1-77.3) | 71 (49-109.4) | 0.356 |
| CRE > 195 μmol/L | 0 | 5 (0.08) | 0.182 |
| FOB | 14 (0.7) | 37 (0.62) | 0.386 |
| CRP (mg/L) (IQR) | 69.5 (9.0 - 133) | 16.25 (5.0 - 49.62) | 0.01 |
| IDSA/SHEA severity | |||
| Severe | 4 (0.23) | 9 (0.15) | 0.600 |
| Mild/moderate | 13 (0.77) | 51 (0.85) | 0.724 |
| Medications during 14 days prior to infection | |||
| Any immunosuppressive treatment | 0 (0) | 4 (0.07) | 0.066 |
| COR | 1 (0.05) | 19 (0.32) | 0.017 |
| PPI | 15 (0.75) | 39 (0.65) | 0.582 |
| Any antibiotic | |||
| No. of antibiotics (≥ 2) | 3 (0.15) | 10 (0.16) | 0.861 |
| Aminoglycosides | 0 (0) | 0 (0) | |
| β-lactam/β-lactamase inhibitor combinations | 7 (0.35) | 16 (0.26) | 0.573 |
| Carbapenems | 3 (0.15) | 6 (0.10) | 1 |
| Third/fourth generation cephalosporins | 3 (0.15) | 5 (0.08) | 0.043 |
| Fluoroquinolones | 7 (0.35) | 12 (0.2) | 0.697 |
| Glycopeptide | 3 (0.15) | 3 (0.05) | 0.141 |
| Tetracyclines | 0 (0) | 2 (0.03) | 0.308 |
| CDI treatment | |||
| Metronidazole alone | 4 (0.23) | 5 (0.08) | 0.242 |
| Vancomycin alone | 7 (0.35) | 10 (0.16) | 0.122 |
| Combination therapy | 6 (0.3) | 6 (0.1) | 0.025 |
| Outcomes | |||
| Length of hospital stay (IQR) | 14.5 (9 - 21.5) | 15 (7 - 29) | 0.794 |
| All-cause in-hospital mortality | 3 (0.15) | 7 (0.11) | 0.696 |
Abbreviations: IQR, interquartile range; CDI, Clostridium difficile infection; PPI, proton-pump inhibitor; COR, cortisol; WBC, white blood cell count; NEU, neutrophile granulocyte; Hb, hemoglobin; PLT, blood platelet count; ALB, albumin; AST, glutamate aspartate transaminase; CRE, creatinine; CRP, C-reactive protein; FOB, fecal occult blood; SHEA, Society for Healthcare Epidemiology of America; IDSA, Infectious Diseases Society of America.
a Values are presented as No. (%) or mean ± SD.
Out of the 80 patients, 9 cases received only metronidazole alone, 17 cases were treated with vancomycin alone, and 12 cases received a combination therapy of both drugs. There was a significant difference between the NAP1 and non-NAP1 groups regarding the combination therapy (6 vs. 6, P = 0.025). However, there was no significant difference in all-cause in-hospital mortality rates between the NAP1/027 group (15%) and non-NAP1/027 group (11%) (P = 0.696). The average length of hospitalization was also similar between the two groups (14.5 vs. 15 days, P = 0.794). According to univariate logistic regression analysis, hospitalization in the 90 days before the CDI diagnosis (OR = 8.419; 95% CI, 1.794 - 39.514) and high CRP levels within 3 days of CDI diagnosis (OR = 1.008, 95% CI, 1.001 - 1.016) were identified as risk factors for NAP1 CDI (Table 2).
| Variable | OR | 95% CI | P-Value |
|---|---|---|---|
| Risk factors of CDI in patients with NAP1/027 | |||
| Hospitalization in the 90 days before CDI diagnosis | 8.419 | 1.794 - 39.514 | 0.007 |
| High CRP level within ± 3 days of C. difficile detection | 1.008 | 1.001 - 1.016 | 0.036 |
Abbreviations: CDI, Clostridium difficile infection; CI, confidence interval; CRP, C-reactive protein; OR, odds ratio
5. Discussion
Several NAP1/027 CDI cases have been reported in European, UK, and Asian countries (13, 17, 18). Despite the emergence of CDI as a problem in China, few studies have examined the infection-associated risk factors in this population (19, 20). Moreover, the data on the specific risk factors and clinical outcomes of C. difficile isolates are scarce. To the best of our knowledge, this study represents the first investigation in China to identify the risk factors and clinical outcomes associated with CDI caused by NAP1/027 strains. It is debatable whether NAP1/027 CDI is associated with disease severity or mortality. Previous research has suggested that CDI patients induced by NAP1/027 strains might experience more severe outcomes than those diagnosed due to the non-NAP1/027 strain (21). However, some studies have contradicted this finding and suggested that NAP1/027 strains do not always result in more severe outcomes, despite producing larger amounts of toxins (8). The present study observed a NAP1/027 prevalence of 15.07%, lower than other studies reporting a positivity rate ranging from 18% to 24% (8, 10). However, no obvious differences were observed in disease severity, length of hospitalization, or mortality between the NAP1/027 and non-NAP1/027 groups.
Advanced age, CDI in the past 12 months, residence in a skilled nursing facility, previous use of fluoroquinolones, and PPI use in the preceding month have been identified as risk factors for the onset of CDI due to NAP1 strains (22, 23). In the present study, hospitalization in the 90-day period prior to the CDI diagnosis and a high CRP level within 3 days of C. difficile diagnosis were significant risk factors for the development of CDI due to NAP1/027 strains. The present study’s findings are consistent with other published reports that identified previous hospitalization as one of the most prominent risk factors for CDI development (20). Additionally, several reports have demonstrated an association between CRP and CDI (24). Serum CRP levels could be used as a biomarker for estimating the likelihood of recurrence and mortality in patients with C. difficile-associated diarrhea (25). Elevated CRP levels have been identified as a significant risk factor for severe CDI, and they can also be used as an indicator of inadequate response to metronidazole in patients with mild-to-moderate CDI (26). The prognostic value of serum inflammatory markers, such as CRP, can guide clinicians in diagnosing CDI caused by NAP1/027 strains earlier and control nosocomial infection and outbreak.
In this study, no significant difference was observed in the use of fluoroquinolones between the NAP1/027 and non-NAP1/027 groups. Although some studies have suggested that fluoroquinolone use is an important risk factor for the development of CDI due to NAP1/027 strains, the evidence is not conclusive. The relationship between fluoroquinolone use and CDI due to NAP1/027 strains might be attributed to the frequency and duration of use or specific characteristics of the drug class (27). Further research is needed to better understand this relationship.
It is important to note that the retrospective design of the study and the small sample size limited the generalizability of the findings to other populations and settings. In addition, the absence of relapse information during follow-up prevented statistical testing of risk factors for CDI recurrence, which could have been informative. Furthermore, multivariate regression analysis was not conducted to avoid the overestimation of the effect size. Therefore, further studies with prospective designs and larger sample sizes are warranted to verify the current study’s findings and identify other potential risk factors for CDI due to NAP1/027 strains.
5.1. Conclusions
This study demonstrated both similar and divergent findings, compared to previous research, on patients with CDI due to NAP1/027 strains. Notably, the obtained findings highlight the potential usefulness of the serum CRP level as a prognostic factor in these patients. However, due to the limitations of this small-scale retrospective study, further research on a larger scale is necessary to gain a deeper understanding of the risk factors associated with the NAP1 strain and the relationship between this strain and clinical outcomes, with the ultimate goal of improving early diagnosis and infection control.