1. Background
Human rhinovirus (HRV) and human metapneumovirus (hMPV) are the leading causative agents of acute respiratory tract infections (ARIs) in humans throughout the world, regardless of age group (1-5). In China, the reported frequency of HRV infection in children was 4.79% - 27.4%, while the frequency of hMPV infection was about 1.5% - 7.9% (3-5). Both infections with HRV and hMPV can cause a variety of respiratory tract infections, ranging from mild upper respiratory tract disease, i.e., laryngitis, to tracheitis, bronchitis, and severe pneumonitis. While their clinical manifestations may overlap, there are likely differences in symptoms and severity between HRV and hMPV alone. It is not unusual for both bacterial and viral pathogens to be present during infections of the respiratory system that cause conditions like pneumonia, bronchitis, and the common cold (6, 7). HRV and hMPV are often found to co-infect with other respiratory pathogens (2, 4). However, the clinical characteristics and manifestations of these viral-bacterial co-infections are not well understood. In particular, the specific interactions between pathogens and their contribution to disease severity need to be further elucidated through research.
2. Objectives
The present study aimed to analyze and compare the clinical features of HRV and hMPV mono-infections in hospitalized children, evaluate the rates of bacterial co-detection in HRV and hMPV infections, assess the impact of bacterial co-infection on the disease severity of HRV and hMPV infections, and analyze differences in disease characteristics and outcomes between viral mono-infections and viral-bacterial co-infections among hospitalized pediatric patients.
3. Methods
3.1. Patient Selection
This study retrospectively enrolled hospitalized children aged 14 years or younger with ARIs from the Maternal and Child Health Hospital of Hubei Province in Wuhan between January and December 2022. ARIs were categorized as pneumonia, bronchitis, or upper respiratory infection based on clinical symptoms, physical examination findings, and chest X-ray results where available. Specifically, pneumonia was defined as the presence of fever, cough, tachypnea, breathing difficulties, and lung infiltrates on chest X-rays. Bronchitis was defined as cough, wheezing, rhonchi, and absence of infiltrates on chest X-rays.
Upper respiratory infections were defined as rhinorrhea, pharyngitis, and absence of auscultatory findings and infiltrates on chest X-rays when performed. Disease severity between the different infection groups was determined based on several predefined criteria, including the incidence of pneumonia, the utilization of mechanical ventilation, the rate of admission to the pediatric intensive care unit (PICU), and the increased duration of hospitalization. We analyzed digital clinical data, including demographic, epidemiological, diagnostic, and laboratory information.
3.2. Respiratory Virus Detection
Nasopharyngeal secretions or alveolar lavage fluid collected within 24 hours after admissions were analyzed. A panel of respiratory viruses, including influenza A and B, respiratory syncytial virus (RSV), human parainfluenza virus, HRV, hPMV, human coronaviruses (NL63, OC43, 229E, and HKU1), human adenovirus, human bocavirus (HBoV), Mycoplasma pneumonia, and Chlamydia, were detected in these patients using commercial polymerase chain reaction (PCR) capillary electrophoresis kits (Ningbo Haiers Gene Technology Co., Ltd., China).
3.3. Microbial Culture and Identification
Bacterial co-infections were identified through evidence from sterile sites, such as bronchoalveolar lavage, or nonsterile sites, like sputum and nasopharyngeal swabs.
Twenty-four hours after admission, microbial culture and identification processes were carried out in accordance with the routine diagnostic standard operating procedures applied in the clinical laboratory of this hospital: Bronchoalveolar lavage or sputum was collected from patients suspected of ARI and then cultured. Blood, chocolate, and MacConkey plates were employed for the inoculation of the samples. The blood and chocolate plates were incubated for 72 hours or until observing a positive result in a carbon dioxide incubator at a concentration of 5 - 10%. The MacConkey plate was also put into an incubator at 35°C - 37°C for 24 to 48 hours. Afterward, Bruker matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry was used to determine positive cultures at the species level (Bruker Daltonik GmbH, Leipzig, Germany).
3.4. Exclusion Criteria
The exclusion criteria in the present research included (1) patients with chronic pulmonary diseases possibly influencing the chest X-ray results, aspiration pneumonia, or interstitial lung disease; (2) patients with compromised immune systems or those taking immunosuppressive medications; (3) patients with a suspected nosocomial or fungal infection; and (4) patients with insufficient clinical data.
3.5. Statistical Analysis
Statistical analyses were performed using SPSS software version 21.0 (SPSS, Inc., Chicago, IL, USA). Comparisons of the frequencies among groups were conducted using the chi-square test or Fisher’s exact test. The independent sample t-test was used to compare the mean values between groups. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Comparison of the Clinical Data Between Human Rhinovirus and Human Metapneumovirus Mono-infections
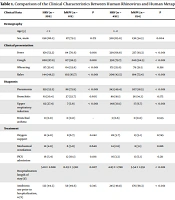
Between January and December 2022, we investigated 1,978 children hospitalized with HRV infection, of which 1,529 had HRV mono-infection and 1,117 were hospitalized with hMPV infection, among whom 910 had hMPV mono-infection. As demonstrated in Table 1, the male proportion of patients with HRV mono-infection was greater (61.4%, 939/1529) than that of patients with hMPV mono-infection (54.9%, 500/910) (P = 0.002). Compared to HRV, hMPV mono-infection exhibited more pronounced symptoms of fever, cough, and rales in most age groups, while HRV showed more wheezing. Except in patients ≥ 6 years old, hMPV was more associated with pneumonia and longer hospitalizations.
| Clinical Data | HRV (n = 299) | hMPV (n = 119) | P | HRV (n = 492) | hMPV (n = 254) | P | HRV (n = 580) | hMPV (n = 501) | P | HRV (n = 158) | hMPV (n = 36) | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demography | ||||||||||||
| Age (y) | < 1 | 1 - 2 | 3 - 5 | ≥ 6 | ||||||||
| Sex, male | 198 (66.2) | 87 (73.1) | 0.172 | 320 (65.0) | 138 (54.3) | 0.004 | 335 (57.8) | 255 (50.9) | 0.024 | 86 (54.4) | 20 (55.6) | 0.903 |
| Clinical presentation | ||||||||||||
| Fever | 159 (53.2) | 84 (70.6) | 0.001 | 339 (68.9) | 237 (93.3) | < 0.001 | 397 (68.4) | 471 (94.0) | < 0.001 | 108 (68.4) | 28 (77.8) | 0.265 |
| Cough | 260 (87.0) | 117 (98.3) | 0.001 | 392 (79.7) | 240 (94.5) | < 0.001 | 486 (83.8) | 479 (95.6) | < 0.001 | 121 (76.6) | 34 (94.4) | 0.029 |
| Wheezing | 97 (32.4) | 64 (53.8) | < 0.001 | 172 (35.0) | 79 (31.1) | 0.291 | 149 (25.7) | 82 (16.4) | < 0.001 | 25 (15.8) | 4 (11.1) | 0.648 |
| Rales | 144 (48.2) | 102 (85.7) | < 0.001 | 209 (42.5) | 184 (72.4) | < 0.001 | 224 (38.6) | 340 (67.9) | < 0.001 | 48 (30.4) | 13 (36.1) | 0.504 |
| Diagnosis | ||||||||||||
| Pneumonia | 159 (53.2) | 88 (73.9) | < 0.001 | 243 (49.4) | 207 (81.5) | < 0.001 | 296 (51.0) | 399 (79.6) | < 0.001 | 79 (50.0) | 31 (86.1) | < 0.001 |
| Bronchitis | 61 (20.4) | 27 (22.7) | 0.605 | 89 (18.1) | 36 (14.2) | 0.175 | 93 (16.0) | 64 (12.8) | 0.129 | 22 (13.9) | 2 (5.6) | 0.273 |
| Upper respiratory infection | 82 (27.4) | 7 (5.9) | < 0.001 | 148 (30.1) | 17 (6.7) | < 0.001 | 179 (30.9) | 40 (8.0) | < 0.001 | 55 (34.8) | 4 (11.1) | 0.010 |
| Bronchial asthma | 0 (0.0) | 0 (0.0) | - | 3 (0.6) | 0 (0.0) | 0.555 | 16 (2.8) | 4 (0.8) | 0.031 | 9 (5.7) | 1 (2.8) | 0.766 |
| Treatment | ||||||||||||
| Oxygen support | 12 (4.0) | 8 (6.7) | 0.242 | 28 (5.7) | 13 (5.1) | 0.745 | 27 (4.7) | 8 (1.6) | 0.005 | 10 (6.3) | 0 (0.0) | 0.213 |
| Mechanical ventilation | 12 (4.0) | 6 (5.0) | 0.640 | 14 (2.8) | 8 (3.1) | 0.816 | 11 (1.9) | 0 (0.0) | 0.001 | 2 (1.3) | 2 (5.6) | 0.325 |
| PICU admission | 16 (5.4) | 12 (10.1) | 0.081 | 16 (3.3) | 13 (5.1) | 0.211 | 14 (2.4) | 2 (0.4) | 0.013 | 2 (1.3) | 0 (0.0) | 1.000 |
| Hospitalization length of stay (d) | 5.18 ± 2.668 | 6.03 ± 3.310 | 0.007 | 4.83 ± 1.780 | 5.54 ± 1.951 | < 0.001 | 4.86 ± 1.879 | 5.26 ± 1.541 | < 0.001 | 5.00 ± 3.109 | 5.08 ± 2.143 | 0.879 |
| Antibiotic use prior to hospitalization, n (%) | 133 (44.5) | 59 (49.6) | 0.345 | 245 (49.8) | 176 (69.3) | < 0.001 | 340 (58.6) | 376 (75.0) | < 0.001 | 107 (67.7) | 25 (69.4) | 0.841 |
Abbreviations: HRV, human rhinovirus; hMPV, human metapneumovirus; PICU, pediatric intensive care unit.
a Values are expressed as No. (%) or mean ± SD.
4.2. Bacterial Co-pathogens Detected in Human Rhinovirus and Human Metapneumovirus Infections
Among the 1,978 children infected with HRV, 63 (3.2%) had bacterial co-infections. Of the 1,117 children infected with hMPV, 49 (4.4%) had bacterial co-infections. In hMPV co-infected cases, Streptococcus pneumoniae had the highest detection rate (n = 36, 73.5%), followed by Haemophilus influenzae (n = 11, 22.4%) and Pseudomonas aeruginosa (n = 2, 4.1%). The co-infection rate of hMPV and S. pneumoniae was significantly higher than that of HRV (P = 0.015). The bacterial pathogens detected in co-infections are summarized in Table 2.
| With Bacteria | HRV (n = 63) | hMPV (n = 49) | P |
|---|---|---|---|
| Total infection cases | 63 (3.2) | 49 (4.4) | 0.086 |
| Streptococcus pneumoniae | 32 (50.8) | 36 (73.5) | 0.015 |
| Klebsiella pneumoniae | 2 (3.2) | 0 (0.0) | 0.503 |
| Staphylococuus aureus | 5 (7.9) | 0 (0.0) | 0.067 |
| Haemophilus influenzae | 23 (36.5) | 11 (22.4) | 0.108 |
| Pseudomonas aeruginosa | 1 (1.6) | 2 (4.1) | 0.825 |
Abbreviations: HRV, human rhinovirus; hMPV, human metapneumoviru.
a Values are expressed as No. (%).
4.3. Comparison of Clinical Data Between Patients with Human Rhinovirus Mono-infection and Co-infection
We conducted statistical analysis on various clinical data of patients with HRV mono-infection and co-infection (Table 3). There was a statistical difference in the age distribution between HRV mono-infection and HRV co-infection with bacteria (P = 0.002). Children with bacterial co-infections had a higher proportion of coughs (P < 0.001), rales (P = 0.002), pneumonia (P < 0.001), PICU admissions (P < 0.001), and longer hospitalizations (P = 0.003).
| Clinical Data | With Bacteria (n = 63) | Mono-infection (n = 1529) | P |
|---|---|---|---|
| Demography | |||
| Sex, male | 41 (65.1) | 939 (61.4) | 0.558 |
| Age (y) | 0.002 | ||
| < 1 | 13 (20.6) | 299 (19.6) | |
| 1 - 2 | 9 (14.3) | 492 (32.2) | |
| 3 - 5 | 27 (42.9) | 580 (37.9) | |
| ≥ 6 | 14 (22.2) | 158 (10.3) | |
| Clinical presentation | |||
| Fever, No. (%) | 37 (58.7) | 1003 (65.6) | 0.262 |
| Cough, No. (%) | 63 (100.0) | 1259 (82.3) | < 0.001 |
| Wheezing | 22 (34.9) | 443 (29.0) | 0.309 |
| Rales | 37 (58.7) | 625 (40.9) | 0.005 |
| Diagnosis | |||
| Pneumonia | 48 (76.2) | 777 (50.8) | < 0.001 |
| Bronchitis | 10 (15.9) | 265 (17.3) | 0.764 |
| Upper respiratory infection | 9 (14.3) | 464 (30.3) | 0.006 |
| Bronchial asthma | 2 (3.2) | 28 (1.8) | 0.767 |
| Treatment | |||
| Oxygen support | 4 (6.3) | 77 (5.0) | 0.863 |
| Mechanical ventilation | 4 (6.3) | 39 (2.6) | 0.154 |
| PICU admission | 8 (12.7) | 48 (3.1) | < 0.001 |
| Hospitalization length of stay (d) | 6.24 ± 3.402 | 4.93 ± 2.181 | 0.003 |
| Antibiotic use prior to hospitalization | 35 (55.6) | 825 (54.0) | 0.803 |
Abbreviation: PICU, pediatric intensive care unit.
a Values are expressed as No. (%) or mean ± SD.
4.4. Comparison of Clinical Data Between Patients with Human Metapneumovirus Mono-infection and Patients with Co-infection
As shown in Table 4, demographic characteristics, clinical presentation, diagnosis, and treatments showed no significant difference between patients with hMPV mono-infection and those with co-infection.
| Clinical Data | With Bacteria (n = 49) | Mono-infection (n = 910) | P |
|---|---|---|---|
| Demography | |||
| Sex, male | 24 (49.0) | 500 (54.9) | 0.414 |
| Age (y) | |||
| < 1 | 6 (12.2) | 119 (13.1) | 0.230 |
| 1 - 2 | 19 (38.8) | 254 (27.9) | |
| 3 - 5 | 21 (42.9) | 501 (55.1) | |
| ≥ 6 | 3 (6.1) | 36 (4.0) | |
| Clinical presentation | |||
| Fever | 42 (85.7) | 820 (90.1) | 0.320 |
| Cough | 48 (98.0) | 870 (95.6) | 0.666 |
| Wheezing | 9 (18.4) | 229 (25.2) | 0.283 |
| Rales | 38 (77.6) | 639 (70.2) | 0.273 |
| Diagnosis | |||
| Pneumonia | 43 (87.8) | 725 (79.7) | 0.167 |
| Bronchitis | 4 (8.2) | 129 (14.2) | 0.330 |
| Upper respiratory infection | 3 (6.1) | 68 (7.5) | 0.943 |
| Bronchial asthma | 1 (2.0) | 5 (0.5) | 0.719 |
| Treatment | |||
| Oxygen support | 3 (6.1) | 29 (3.3) | 0.480 |
| Mechanical ventilation | 2 (4.1) | 16 (1.8) | 0.531 |
| PICU admission | 2 (4.1) | 27 (3.0) | 0.988 |
| Hospitalization length of stay (d) | 5.96 ± 2.784 | 5.43 ± 2.009 | 0.196 |
| Antibiotic use prior to hospitalization | 35 (55.6) | 825 (54.0) | 0.803 |
Abbreviation: PICU, pediatric intensive care unit.
a Values are expressed as No. (%) or mean ± SD.
5. Discussion
Compared to HRV, in most age groups, hMPV infection exhibited more severe symptoms like fever, cough, and rales, while HRV manifested more wheezing. The increased pneumonia and hospitalization associated with hMPV suggest it is a generally more severe infection. Previous reports have compared the severity of hMPV infection to other respiratory viruses, such as HBoV and RSV, based on indicators including pneumonia incidence, mechanical ventilation use, PICU admission rates, and hospitalization duration. These studies have suggested that hMPV causes less severe illness than HBoV but more severe illness than RSV (8, 9).
The reasons for these differences are likely attributable to variations in viral properties and immune responses between hMPV, HBoV, and RSV. Specifically, one study characterized the relationship between neonatal rhinovirus infection and type 2 inflammation in a neonatal HRV model. In contrast, another study found that hMPV-infected children had increased T-helper type 1 (Th1) responses compared to controls (10, 11). Thus, differences in immune responses elicited by different respiratory viruses, as evidenced by these studies, may contribute to variations in clinical severity. Further research is needed to elucidate the viral and immunological factors underlying the spectrum of disease severity caused by common pediatric respiratory viruses.
Compared to previous literature, the bacterial co-infection rate in this study is lower than reports from other regions (8, 12), which can be due to several possible reasons:
- The present investigation was conducted in 2022. Due to the coronavirus disease 2019 (COVID-19) pandemic, the Wuhan government still had relatively strict control measures during this period, with less population mobility, and local residents took more stringent personal protective measures. In fact, the co-infection rate in our study is only slightly lower than other studies from the same time period (13).
- Antibiotic use was prevalent among patients before hospitalization.
- There were differences in epidemiological characteristics across regions.
- Some patients with respiratory symptoms did not undergo bacterial pathogen testing.
While HRV also had a high co-infection rate with S.pneumoniae at 50.8%, hMPV demonstrated an even higher rate at 73.5%, indicating a particular affinity between hMPV and S. pneumoniae. Possible factors include hMPV more extensively damaging airways and impairing mucociliary clearance compared to HRV, allowing greater bacterial adhesion (14, 15). It is possible that hMPV also induces defects in specific immune pathways that favor S. pneumoniae infection (15). Streptococcus pneumoniae infection could potentially enhance hMPV replication to a greater degree as well (16). The differences in co-infection rates suggest variations in viral properties that specifically promote synergism with S. pneumoniae.
The impact of co-infection on ARI severity remains controversial (17, 18). A previous study did not find significant clinical differences between HBoV infection and HBoV co-infection (17). However, another study reported increased PICU admission rates associated with RSV and bacterial co-infection (18). The discrepant findings indicate that the effects of co-infection may depend on the specific viruses and bacteria involved. Further research is needed to clarify which combinations of viruses and bacteria lead to more severe clinical manifestations. Carefully designed studies comparing clinical characteristics of sole viral infections, sole bacterial infections, and specific viral-bacterial co-infections are warranted. Such research will help elucidate the underlying mechanisms of disease exacerbation by viral-bacterial co-infections in pediatric ARIs.
In our study, children co-infected with HRV and bacteria had more severe illnesses compared to those with HRV infection alone. The co-infected group had significantly higher rates of cough, rales, pneumonia, PICU admissions, and longer hospitalizations. This is possibly due to bacterial infections increasing airway inflammation caused by HRV through the release of endotoxins and cytokines (19, 20). In contrast, though hMPV causes more severe illnesses in children than HRV, bacterial co-infections do not appear to worsen hMPV disease. One hypothesis is that the cytotoxic effects induced by hMPV infection on the respiratory epithelium may play a more important role in determining disease severity, outweighing additional damage due to bacterial co-pathogens (21), which differs from HRV-bacterial co-infections, where synergistic interactions between virus and bacteria further impair respiratory tract defense mechanisms (22).
Co-infections may worsen hMPV disease in high-risk subgroups, similar to those with underlying conditions. However, current evidence suggests that preventing bacterial co-infections may not improve outcomes for otherwise healthy children with hMPV. The viral pathogenesis itself appears most important, which highlights key differences between hMPV and HRV and shows that bacterial co-infections cannot always be assumed to worsen viral respiratory illnesses. More research is required on the complex interplay between specific viruses, bacteria, and the host immune response.
This study has several limitations. First, the retrospective design meant that certain potential confounding variables, including household crowding, recent contact with sick siblings or other individuals with respiratory symptoms, and passive smoke exposure, were not systematically captured in the medical records, which may have influenced the findings. Second, a high proportion of children received antibiotics prior to hospitalization. Though antibiotic usage rates were comparable across most control groups, this pretreatment could have introduced bias into the statistical analyses. Third, missing respiratory pathogen data for a subset of patients due to lack of testing led to incomplete epidemiologic data. Finally, the data collected during the COVID-19 epidemic prevention stage may only reflect pathogen prevalence patterns specific to this time period.
5.1. Conclusions
In conclusion, hMPV infections in hospitalized children appear to be more severe than HRV infections. Bacterial co-infections with HRV, but not hMPV, aggravate disease.