1. Background
In December 2019, a novel coronavirus was first reported in Wuhan City, Hubei Province, China. The World Health Organization (WHO) declared it a pandemic in March 2020 (1, 2). COVID-19, caused by SARS-CoV-2, was the most lethal and pervasive pandemic in recent decades, significantly impacting people's lives, health, medicine, and the economy (3). COVID-19 has caused more than 560 million cases and more than 6.3 million deaths worldwide (4). SARS-CoV-2 can manifest with various symptoms, including minor respiratory infections, pneumonia, and serious health issues (such as acute respiratory distress syndrome [ARDS]); also, it can negatively affect the function of vital organs, such as the heart, lungs, and kidneys (5).
People with compromised immune systems or underlying heart illnesses are more likely to die (6, 7). Furthermore, apart from the cardiac complications associated with COVID-19 infection (8), recent studies have indicated the occurrence of new cases of heart failure (HF) in a substantial proportion of individuals admitted to hospitals for COVID-19, even though those cases have no evidence of a previous history of HF (9, 10). Heart failure is a highly prevalent cardiovascular disease (CVD) and a leading cause of mortality worldwide (11). Preexisting and newly acquired HF in the context of the COVID-19 pandemic can pose unique challenges and hinder the diagnosis, treatment, and prediction of outcomes. Early diagnosis of patients exhibiting such symptoms is crucial to facilitate appropriate triage and management strategies.
A significant number of individuals experiencing HF characterized by a decrease in ejection fraction (EF) are typically diagnosed as a result of the complications arising from acute myocardial infarction in the context of ischemic heart disease (IHD) (12, 13). Infection is intricately associated with both the occurrence and worsening of HF. One potential etiology of HF is the direct impact of the virus or the excessive systemic inflammation induced by COVID-19 on cardiac function (14). Patients diagnosed with COVID-19 may encounter hypoxemic respiratory failure, increased myocardial oxygen demand, and disrupted equilibrium between oxygen supply and demand. These factors can either compensate for preexisting HF or give rise to the onset of acute HF (15-17). An additional noteworthy aspect pertains to the increased propensity of individuals afflicted with COVID-19 and CVD to experience myocardial infarction or HF (18-20). Therefore, it is plausible that the COVID-19 infection could worsen preexisting heart disease and potentially lead to the development of new HF.
Recently, the real-time reverse transcription–polymerase chain reaction (RT-PCR) method has emerged as the preferred and widely accepted diagnostic assay for detecting SARS-CoV-2. Nevertheless, various obstacles (such as difficulties in sample collection and transportation, RNA extraction, and the presence of enzyme inhibitors) raise signal serological tests (such as the enzyme-linked immunoassay [ELISA]) to detect immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies; the high-throughput capability is its notable advantage. This advantage helps mitigate false negative cases when relying solely on RT-PCR. In addition, IgG and IgM antibody levels may rise in CVD, such as IHD (21, 22), and both are involved in the diagnosis and severity of COVID-19.
2. Objectives
As an innovative method, we analyzed the blood of patients with HF problems due to COVID-19 to determine the levels of IgG and IgM antibodies essential for the coronavirus spike S protein. The study also examined the potential association between the levels of antibodies and underlying cardiac risk factors in individuals diagnosed with COVID-19.
3. Methods
3.1. Patients
A total of 600 medical records of COVID-19 patients were reviewed from Razi and Golestan hospitals in Ahvaz City, Iran, in the autumn and winter of 2021. Nasopharyngeal and throat swabs were applied to detect respiratory pathogens. All patients diagnosed with COVID-19 were affirmed via real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR) and computed tomography (CT) scans. The diagnosis of all patients regarding epidemic evidence, clinical presentation, chest CT, and laboratory results was made based on the fifth edition guideline on COVID-19 management, which China's National Health Commission set up. Severe COVID-19 patients were defined as individuals who have one of the following signs: respiratory distress with respiratory frequency (RP) ≥ 30/min, pulse oximeter oxygen saturation ≤ 93% at rest, or oxygenation index (arterial partial pressure of oxygen/inspired oxygen fraction [PaO2/FiO2]) ≤ 300 mmHg.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were patients with confirmed COVID-19, exhibiting severe illness, and having experienced 14 to 21 days from the manifestation of symptoms but without prior indications of HF. Patients who showed mild to moderate symptoms of COVID-19 and those who expressed dissatisfaction with the sampling process were excluded based on predetermined criteria. Ultimately, 104 patients were enrolled in the study. The study cohort comprised 52 patients (50%) diagnosed with severe COVID-19. These patients exhibited a reduction in EF to 40% or lower, as determined by echocardiography, in the absence of prior indications of HF. Additionally, these patients had symptoms such as orthopnea and widespread edema. A control group of 52 patients with severe COVID-19, whose EF did not indicate any decline, was chosen.
3.3. Serological Tests
An antibody detection ELISA kit against COVID-19 was used to detect IgG and IgM antibodies against the SARS-CoV-2 virus in human plasma sera. It aims to interact with antigens at the bottom of the well and anti–SARS-CoV-2 antibodies. Anti–SARS-CoV-2 IgG or IgM antibodies bind to the horseradish peroxidase (HRP) enzyme if they are present. The immune complex is produced after washing, eliminating non-specific proteins, and adding a dye solution, the intensity of which is detected by an ELISA reader at a specified wavelength. Hence, a SARS-CoV-2 ELISA kit (94.1% sensitivity, Pishtaz Teb, Iran) was used to detect IgG and IgM antibodies against the SARS-CoV-2 virus in human plasma sera. C-reactive protein (CRP) was also measured using an immunoturbidimetric kit (Biorex Fars, Iran). The target threshold for a positive SARS-CoV-2 infection result was 10 AU/mL.
3.4. Statistical Analysis
SPSS version 21 (SPSS Inc, Chicago, IL, USA) was used for all statistical analyses. The normal distribution is often represented in percentages (%) and continuous quantities, such as medians (interquartile range of 22). The geometric mean (SD) was used to report the antibody level. The Mann-Whitney test or unpaired t-test was used to evaluate the continuous changes. The data were given in terms of the mean and SD (Mean ± SD). P-values less than 0.05 were considered statistically significant.
4. Results
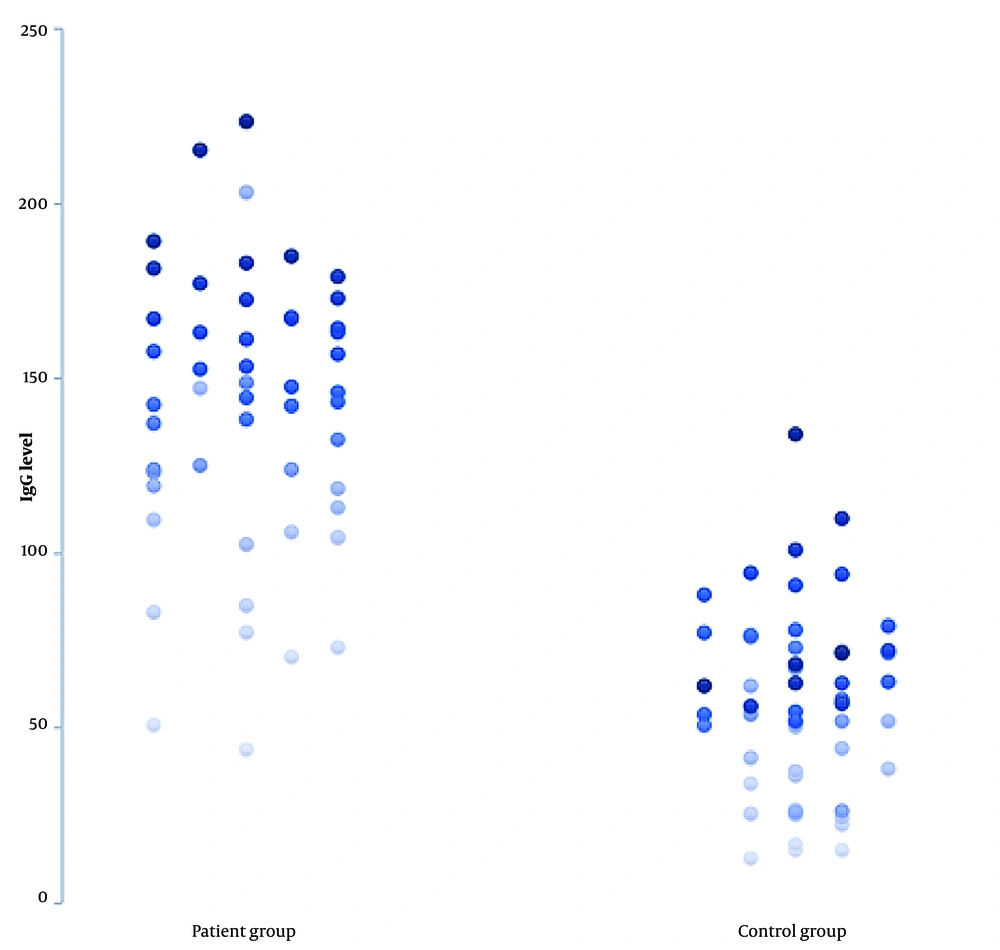
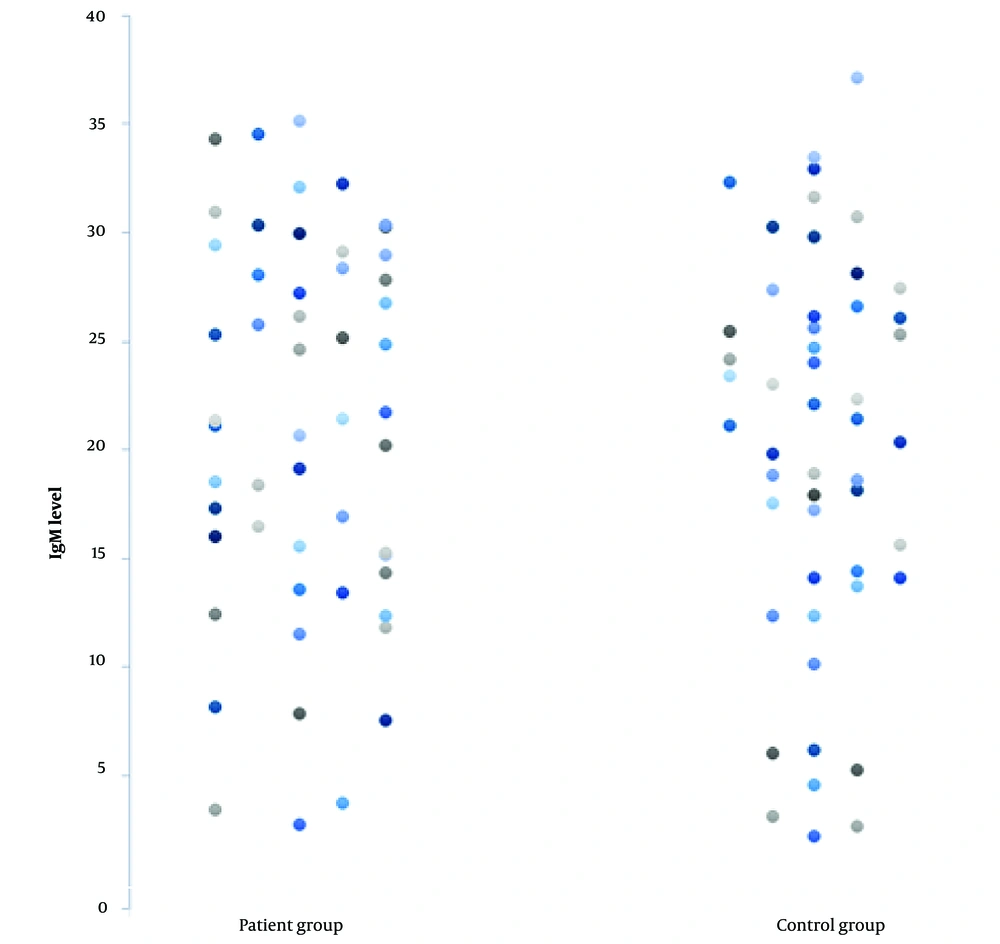
The current study looked over a cohort of 104 hospitalized patients diagnosed with severe or critical cases of COVID-19 (Table 1). The study cohort consisted of 52 patients diagnosed with severe COVID-19. These patients had echocardiographic evidence of decreased EF and showed symptoms such as widespread edema and orthopnea. Simultaneously, no indication of previous HF was found. The control group included 52 individuals diagnosed with COVID-19 with significant problems but were asymptomatic for HF, as determined by echocardiography. The investigation results indicated a substantial difference in IgG levels between patients and controls (Figure 1). Specifically, 86% of HF patients (45 out of 52) exhibited IgG levels above 100 AU/mL, while only 5% (3 out of 52) of the control group had similar levels (P < 0.05). In addition, it is noteworthy that among the 52 patients with severe COVID-19, a significant proportion (42%) had IgG levels over 150 AU/mL. Conversely, the control group did not demonstrate IgG levels at or above this threshold (P < 0.05). However, no significant difference was found in IgM levels between the patient and control groups (P > 0.05; Figure 2).
| Characteristics | Patient Group | Control Group |
|---|---|---|
| All samples | 52 (50) | 52 (50) |
| Age, y | 68 ± 10 | 64 ± 12 |
| Gender (male) | 29 (55) | 27 (51) |
| Temperature, °C | 37 ± 1.0 | 37.2 ± 0.7 |
| Fever, °C | 37 | 40 |
| Oxygen saturation, % | 90 ± 4 | 88 ± 4 |
| Pulse rate, BPM | 112 ± 20 | 101 ± 16 |
| Respiratory rate | 24 ± 6 | 27 ± 7 |
| Dyspnea | 46 (88) | 48 (92) |
| Cough | 32 (61) | 41 (78) |
| Edema | 27 (51) | 4 (0.07) |
| Rales in auscultation | 31 (59) | 5 (0.09) |
a Values are expressed as No. (%) or Mean ± SD.
The level of IgG in COVID-19 patients with a new onset of HF (the patient group) is higher than in COVID-19 patients without HF (the control group). P-values less than 0.05 were considered statistically significant. There is no statistically significant difference in IgM levels between the patient and control groups.
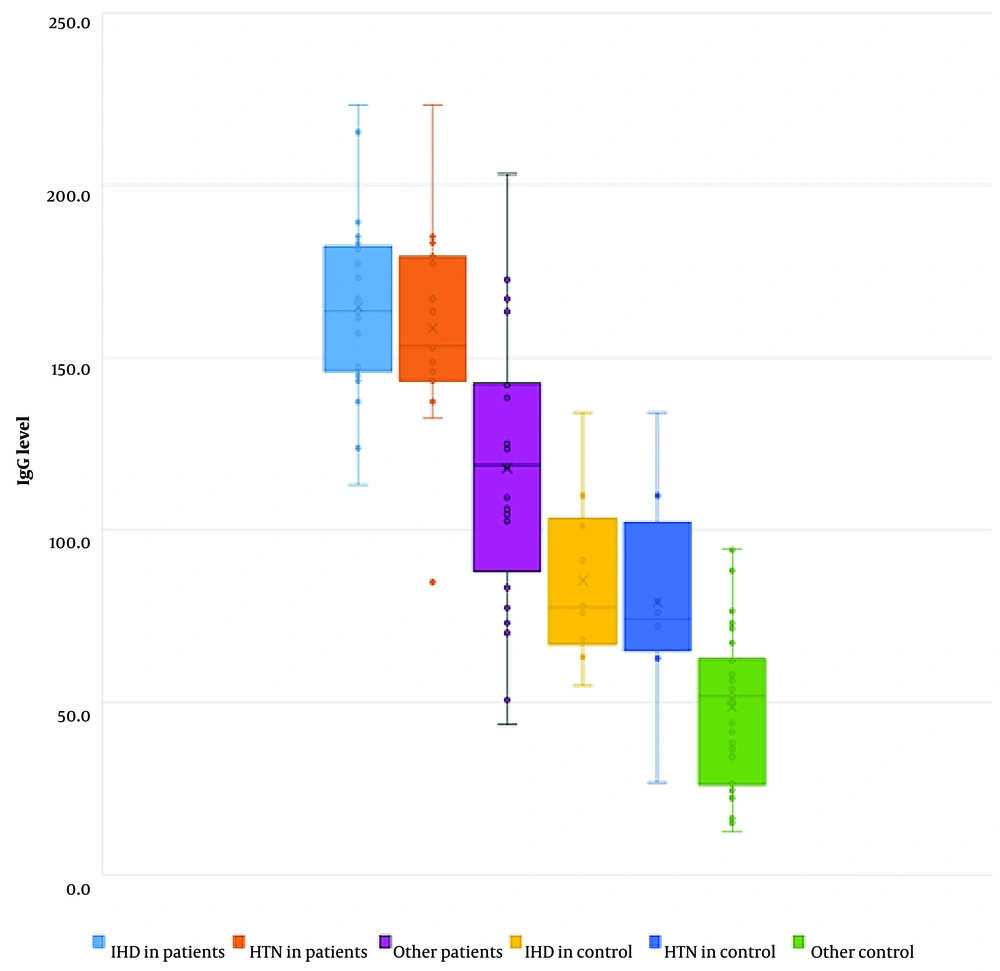
An examination of the medical records of the patient group revealed that the predominant subgroup consisted of individuals with a prior diagnosis of IHD, including 27 out of 48 (56%) patients exhibiting IgG levels above 75 AU/mL. Following that, hypertension was reported in 15 out of 48 cases (31%) and smoking in 13 out of 48 patients (27%; P < 0.05). The IgG levels above 75 AU/mL in the control group demonstrated a similar situation; IHD was prevalent among 6 out of 12 participants, accounting for 50% of the group. Subsequently, hypertension and smoking were other common factors observed in 4 out of 12 patients (25%; P < 0.05; Figure 2). Ischemic heart disease emerged as the prevailing ailment in individuals afflicted with HF during their COVID-19 infection, accounting for 53% of cases. Furthermore, this particular cohort of patients exhibited the greatest concentration of IgG antibodies, surpassing 100 AU/mL, with a prevalence of 96% (P < 0.05). Diabetes was identified as the second comorbidity, affecting 18 out of 52 individuals (34%). Among the subgroup of patients with diabetes, 14 out of 18 (77%) exhibited IgG levels below 75 AU/mL, which was shown to be statistically significant (P < 0.05; Figure 3). The statistical analysis conducted on the levels of IgM and CRP did not provide significant results in both the patient and control groups.
In both the patient and control groups, immunoglobulin G (IgG) levels are more significant in patients with IHD and hypertension than in other subjects. The level of IgG is significantly higher in HF patients with IHD and hypertension than those in controls without HF and with IHD and hypertension.
5. Discussion
The rapid transmission of SARS-CoV-2 led to extensive morbidity and death worldwide. Efforts were made to expedite the discovery of the pathophysiology of the virus, improve the accuracy of diagnosis, and create therapeutic techniques. The clinical presentations and degree of illness seen in people affected with COVID-19 exhibited significant heterogeneity, including a spectrum that spanned from asymptomatic instances to the onset of organ dysfunction (6, 23). Therefore, an accurate and timely diagnosis is of great importance. The standard diagnostic protocols include many methods, such as clinical observations, CT scans, and molecular and serological testing (24-26). Real-time quantitative RT-PCR is the most reliable technique in detecting SARS-CoV-2 infection. However, it is essential to consider several limitations associated with this technique, including the potential for false negative findings, variations in diagnostic accuracy during the progression of the illness, and limited availability of necessary testing materials. Therefore, there has been significant interest in using serological assays as a potential alternative or supplement to RT-PCR for diagnosing acute infection (27-29). Serological assays typically include the measurement of 2 antibodies, namely IgG and IgM (30).
To the best of our knowledge, this research is the first to measure IgG and IgM levels in patients with COVID-19–related HF problems. Patients with new HF during COVID-19 showed a greater IgG level than patients with severe COVID-19 with no indication of HF (control group). Additionally, it was noted that there was a positive correlation between increased IgG levels and the occurrence of new cases of HF. Furthermore, no significant difference was found in IgM levels between the patient cohort and the control group. Typically, the presence of IgG suggests prior exposure to an infection and may be identified 14 - 21 days before the manifestation of symptoms (31). In a recent study, the researchers assessed the IgG antibody levels in a cohort of 37 asymptomatic individuals diagnosed with COVID-19. The findings revealed significantly lower IgG antibody levels in asymptomatic patients than in those with disease symptoms. Additionally, it has been shown that a clear correlation exists between increased levels of antibodies and the level of illness severity. Further, they noted that the presence of IgM, which indicates a recent or active infection, may be identified within 5 - 6 days after the manifestation of symptoms in individuals with symptomatic COVID-19 (32). Another study showed that the diagnostic sensitivity of antibody testing during the first week of COVID-19 infection was less than 30%.
During days 8 - 14, the IgG test exhibited a sensitivity of approximately 66.5%, while the IgM test demonstrated a sensitivity of roughly 58.4%. Notably, a higher sensitivity was observed during the 15 - 21 days following the symptom onset, with the IgG test showing a sensitivity of 88.2% and the IgM test showing a sensitivity of 75.4% (31). In the current study, blood samples were taken within 15 - 21 days following the beginning of symptoms. Patients with severe COVID-19 have been seen to have increased levels of IgG antibodies, suggesting that a robust IgG response may not always correlate with the presence of protective immunity (21). Similar to our analysis, in the study by Xie et al., samples with levels above 10 AU/mL were classified as positive. Their findings are consistent with ours since they identified diabetes (12.5%) and hypertension (5.36%) as the most prevalent underlying diseases in COVID-19 patients (33). Moreover, individuals with preexisting cardiovascular conditions have an increased risk of having severe manifestations of COVID-19 (21).
Due to its heightened specificity toward infections, most serological tests primarily assess the levels of IgG (34). Hence, a serological investigation might be advantageous in examining delayed consequences, performing epidemiological research, and assessing the degree of immune system engagement, especially when RT-PCR assays may provide false negative results because of viral reduction or low viral concentrations in samples (35). Moreover, a study conducted on a sample size of 150 patients revealed that 33% of fatalities attributed to COVID-19 were concomitant with preexisting cardiac illness. In contrast, 7% of deaths were associated with HF (35). The research conducted by van den Hoogen et al. in 2019 revealed an increase in the circulating concentration of IgG, immune cells inside the myocardium, and deposition of IgG in the cardiac tissue, indicating their involvement in the immune response before and during end-stage HF. According to this research, the levels of IgG1 and IgG3 antibodies were considered early predictive markers for HF (21).
Chronic HF (CHF) is defined as evidence of previous congestive decompensation or left ventricular (LV) systolic dysfunction (LV EF less than 40%), as well as acute HF as the sudden development or worsening of HF signs/symptoms (36). In severe COVID-19 patients, HF and cardiac myocarditis are side effects (37). According to the cohort analysis performed by Driggin et al., HF was often found in COVID-19 patients, and it was more prevalent in those who died during hospitalization than in those who survived (17). Furthermore, Rey et al. claimed that there was no information on the impact of COVID-19 on chronic HF patients and its potential to produce acute HF. By investigating 3080 individuals with COVID-19, they revealed that patients with CHF were more prone to acute HF. Furthermore, 77.9% of acute HF patients were found to have no history of HF (36).
Determining the precise rate of cardiac problems in COVID-19 individuals is difficult. Castiello et al. estimated the incidence of myocarditis and HF to be 22 cases per 100 000 people in a 2020 review study. Indeed, since this evaluation only includes reported cases, the true prevalence of HF is greater. This research offered 2 ideas on the etiology of acute myocardial infarction. Two critical assumptions predict angiotensin-converting enzyme 2 (ACE2) receptor direct function and a hyperimmune response. SARS-CoV-2 infiltrates many cells after attaching to ACE2 through its spike protein, including epithelial cells and macrophages. The myocardium also expresses SARS-CoV-2 binding receptors (38). Gheblawi et al. explained that the virus caused HF by inducing a cascade of inflammatory reactions. Based on the limited evidence, the pathophysiological approach may be represented by an immunological response related to the cytokine storm or an intrinsic process starting in the heart (39).
Consequently, the underlying CVD worsens the previously existing heart damage and leads to the development of HF. On the other hand, COVID-19 infection will exacerbate the current cardiac condition and has far-reaching consequences. The most common underlying cause of COVID-19 cardiovascular issues seems to be systemic inflammation (40). Likewise, our data showed that 96% of IgG levels in IHD patients with HF caused by COVID-19 were above 100 AU/mL. These people experienced a significant increase in IgG levels, which might imply an antibody-mediated immune response during heart regeneration. According to a study, IHD is one of the underlying CVDs contributing to HF development (19). Furthermore, the level of IgG increases in these individuals. These antibodies, which are generated in reaction to heart tissue, attach to the failing myocardium and activate complement, which is most likely a key component in the development of HF (24).
As a chronic condition, hypertension represents a pro-inflammatory state (as seen in various infectious diseases), weakened innate immune response, critical element in the pathogenesis of COVID-19 (particularly in critically ill patients). These individuals have increased levels of inflammatory biomarkers, including tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) (41). Furthermore, individuals with hypertension had a more severe COVID-19 disease (42). According to recent studies, 93% of hypertensive individuals with acute HF problems had IgG levels above 100 AU/mL. Cardiovascular patients with COVID-19 had a higher mortality rate, more severe illness, and more comorbidities (such as HF). As a result, it is critical to establish basic approaches for early diagnosis in these individuals. Furthermore, more extensive studies on IgG antibody levels might lead to earlier diagnosis procedures and innovative treatment options for COVID-19–infected cardiac patients.
Although this research is a pilot study conducted during the COVID-19 pandemic, a small sample size is among its limitations. Thus, further studies are needed with larger sample sizes. Evaluating the relationship between different strains of COVID-19 with the level of IgG and heart function is also suggested. Regarding serological tests for COVID-19, the outbreak of the COVID-19 pandemic has led to the process of developing serological kits for laboratory evaluations of patients at an unprecedented pace.
Commercial kits have generally received an Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA). The production process of laboratory kits includes designing, developing, and validation in licensed centers and preliminary evaluation, verification, and maintenance in clinical centers (43). However, this rapid development without considering our main limitation (which is a lack of complete knowledge of the pathophysiology of the disease and the biology of the virus) has uncertainties and shortcomings. For example, the time of seroconversion occurrence for SARS-CoV-2 is not definitely known (44). Since not all patients develop high-titer antibodies, the limitation of detection (LOD) of the serological test is not known with certainty. The mode of interference of other antibodies with phylogenetically related viruses is not known in the current kits (45). Despite the current limitations, we should not underestimate the benefits of serological tests. They can be helpful in diagnosing previous infections when IgG is positive and PCR is negative (due to viral shedding dynamics), which can help to end the individual quarantine of the patient (46) or help to check Herd immunity and general vaccination outcome evaluation (42). With the improvement of emergency conditions, commercial tests will also improve in the near future eventually.
5.1. Conclusions
The incidence of new HF during COVID-19 was higher in patients with IgG levels higher than 100 AU/mL, and a significant increase in IgG level was observed in patients suffering from new HF complications of COVID-19, regardless of previous medical histories or underlying CVD. The findings of this research are notable in that individuals with a history of IHD and hypertension had a tremendous increase in IgG levels. Fewer individuals with additional underlying cardiac illnesses had confirmed HF and higher IgG levels. IgG levels were measured in individuals with a history of IHD, diabetes, smoking, and hypertension who acquired COVID-19 and had significant consequences, including HF, compared to controls. COVID-19 and increased IgG may be a marker of worsening cardiac disease. The increase in IgG was more than usual in cardiac patients whose health had deteriorated.