1. Background
Onychomycosis is the most common fungal infection (1). Onychomycosis affects both fingernails and toenails; this complication is caused by both yeasts and molds (2). The etiological agents of onychomycosis differ in different countries, but according to the brief information in this context in Iran, onychomycosis by Candida species is more common, especially in fingernail infections (2-7). Up to 50% of all nail disorders are related to onychomycosis (1). The prevalence of onychomycosis is related to various factors such as gender, age, occupation, weather, living and traveling environment, and underlying diseases such as diabetes (1, 8).
Paronychia is a common clinical manifestation in onychomycosis by yeasts at the proximal and lateral toenail and fingernail folds and is more common in women than in men, particularly workers in occupations that require them to have their hands or feet submerged in water for prolonged periods (e.g., dishwashers); middle-aged females are at the highest risk of onychomycosis (9). Onychomycosis is not life-threatening, but it can cause complications such as secondary bacterial infection, chronicity, changes in the shape and color of the nail, as well as difficulties related to treatment, and it can often cause social and occupational concerns (10, 11). Therefore, rapid diagnosis of onychomycosis and accurate identification of Candida species is very important because, in these conditions, management and treatment can be successful.
2. Objectives
In this study, we aimed to determine the abundance of candidal onychomycosis of patients referred to the Fasa medical mycology lab, identifying the Candida species using molecular methods and evaluating the in vitro antifungal susceptibility profiles via microdilution broth.
3. Methods
3.1. Clinical Sampling
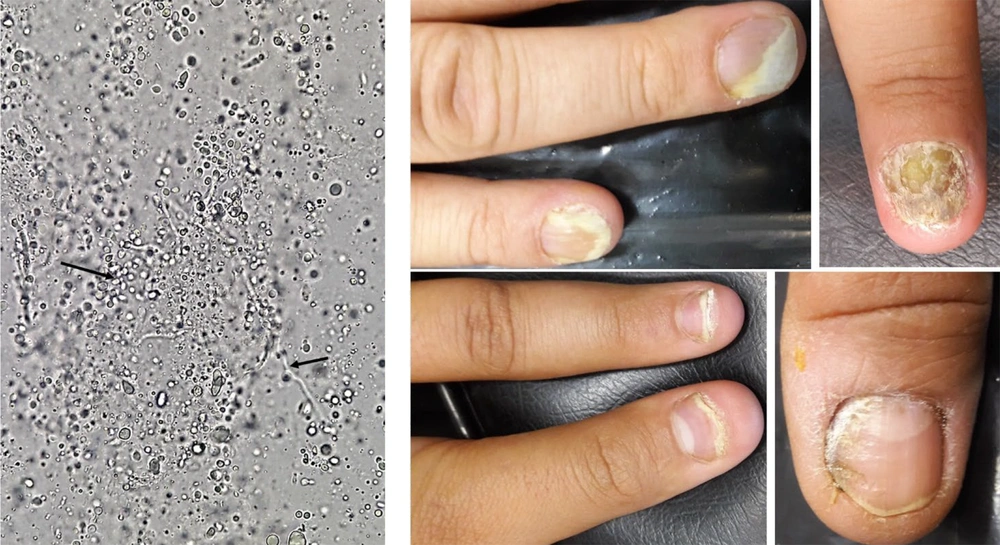
The nails were sampled, including the matrix, bed, or plate that were discolored, thick, fragile, or cracked. In total, 140 clinical specimens were collected from the toenails or fingernails of patients suspected of onychomycosis (age range 3 - 74 years) referring to the medical mycology laboratory of Fasa University of Medical Sciences. The clinical specimens were initially examined by direct microscopic examination (KOH 20%) and then cultured on Sabouraud dextrose agar (SDA, Condalab, Madrid, Spain) supplemented with chloramphenicol and incubated at 35°C for two days. The grown Candida species were introductory identified by CHROMagar Candida medium (CHROMagar Company, Paris, France) (12).
3.2. Molecular Identification
Afterward, isolated Candida species were subjected to molecular identification (PCR-RFLP). Initially, gDNA extraction was done using the glass bead disruption method, then PCR amplification of ITS1-5.8S-ITS2 rDNA regions was performed in a final volume of 25 µL by using the primers ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3’ and ITS4 (5’-TCC TCC GCT TAT TGA TAT GC-3’) (Metabion, Germany) (13, 14). Each reaction consisted of 1µL template DNA, 1 µL of each primer at 10 pmol, 12.5 µL MasterMix containing 5 mM dNTP, 0.5 U Taq DNA polymerase, and 10X PCR buffer (Ampliqon, Denmark) and 9.5 µL double-distilled water. PCR conditions were adjusted with cycles of five minutes at 94°C for primary denaturation, followed by 35 cycles at 94°C (30 seconds), 55°C (45 seconds), and 72°C (60 seconds), and final extension at 72°C for seven minutes. Subsequently, PCR products were digested in a final reaction volume of 30µL containing 17µL water, 2µL of 10X FastDigest buffer, and 1 U of FastDigest restriction enzyme MspI (Fermentas, Thermo Fisher Scientific Inc., USA) and 10 µL PCR product at 37°C for 10 minutes (12, 14).
3.3. In Vitro Susceptibility Testing
The in vitro susceptibility of four triazole antifungal drugs testing of 51 Candida species was based on broth microdilution method using minimum inhibitory concentrations (MICs) following the M27-A3 and M27-S4 guidelines of the Clinical and Laboratory Standards Institute (CLSI) (15, 16). Voriconazole (VRC), itraconazole (ITC) and posaconazole (POS) (All, Sigma-Aldrich, USA) were dissolved in dimethyl sulfoxide (Sigma, Germany) and fluconazole (FLC) (Sigma-Aldrich, USA) in deionized-distilled water. The antifungal agents were diluted in the standard RPMI-1640 medium (Sigma Chemical Co. Germany) buffered to pH 7.0 with 0.165 M-morpholine propane sulfonic acid (MOPS) (Sigma, Germany) with L-glutamine without bicarbonate to yield two times their concentrations. The buffer was dispensed into 96-well microdilution trays at a final concentration of 0.016 - 16 μg/mL for ITC, VRC, and POS and 0.063 - 64 μg/mL for FLC. Briefly, homogeneous yeast suspensions were spectrophotometrically measured at the 530 nm wavelength and a percent transmission within the 75 - 77% range. Therefore, the final densities of the stock inoculum suspensions of the tested isolates ranged between 2.5×103 and 5×103 colony forming units/mL. The 96-well microplates were incubated at 35°C and examined visually after 24 and 48 h to determine MIC values. The Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were quality control strains (15, 16).
4. Results
Generally, out of 140 nail samples, including 71 fingernails (46 female and 25 male) samples, 31 toenail samples (21 female and 10 male), and 38 samples of both fingernail and toenail (15 female and 4 male) were taken from 82 females (F) and 39 males (M). Direct microscopic examination by KOH 20% of 140 nail samples showed that 51 (36.4%) samples (42 female and 7 male) were positive in terms of fungal elements (yeast & budding and pseudohyphae) (Figure 1); also, the cultures of all 51 (36.4%) samples were positive. Out of 51 positive samples (49 patients), 41 (80.4%) samples (41 patients) were fingernail, 6 (11.8%) toenail samples (6 patients), and 4 (7.8%) finger & toe nail samples (2 patients) (Figure 2).
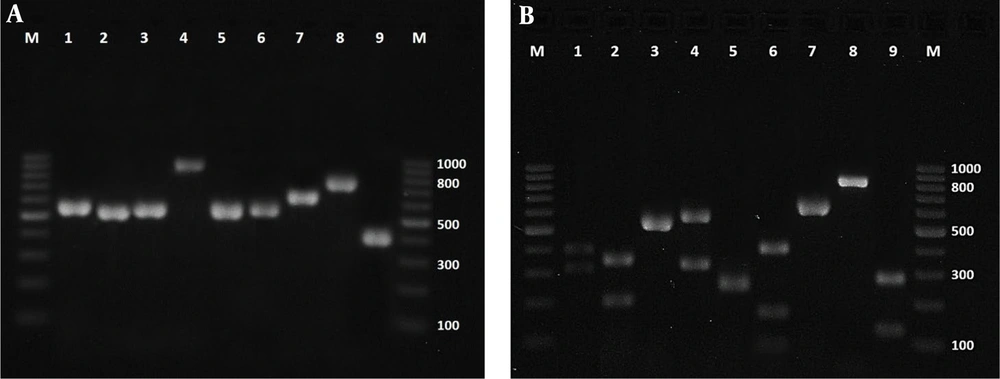
In this study by molecular methods (Figure 3), C. parapsilosis complex (n = 14; 27.5%) was the most frequently isolated of patients (F = 12, M = 2), followed by C. albicans complex (n = 12; 23.5%) (F = 11, M = 1), C. glabrata (n = 6; 11.8%), (F = 5, M = 1), C. krusei (n = 5; 9.8%) (F = 4, M = 1), C. tropicalis (n = 5; 9.8%) (F = 4, M = 1), C. guilliermondii (n = 3; 5.9%), (F = 3), C. famata (n = 3; 5.9%) (F = 2, M = 1), C. kefyr (n = 2; 3.9%) (F = 2) and Candida species (probably, C. rugose, C. intermedia or C. lusitaniae) (n = 1; 1.9 %) (F = 1) (Table 1). The results of in vitro antifungal susceptibility testing of 51 Candida species revealed that all isolates of C. albicans complex, C. tropicalis, C. famata, C. guilliermondii, C. kefyr, and one Candida species were susceptible to four triazoles. Still, out of fourteen C. parapsilosis complexes, a single isolate was resistant to FLC and susceptible dose-dependent (SDD) to ITC and POS. The six C. glabrata isolates were susceptible to VRC and POS, and five of them were susceptible dose-dependent (SDD) to FLC. Still, a C. glabrata was resistant to FLC, and two isolates showed dose-dependent (SDD) to ITC. Also, the five C. krusei isolates were resistant to FLC and susceptible to VRC, but out of five C. krusei, a single isolate was SDD to ITC and POS, and one isolate was resistant to itraconazole (Table 2).
Agarose gel electrophoresis of ITS-PCR products of Candida species (left) and PCR-RFLP after digestion with MspI (right); lanes 1 - 9: Candida albicans complex, C. tropicalis, C. parapsilosis complex, C. glabrata, C. krusei, C. guilliermondii, C. famata, C. kefyr and Candida species (Probably, C. rugose or C. intermedia or C. lusitaniae) respectively; Lane M: A 100-bp DNA size marker.
| Isolate | Total | Fingernail | Toenail | Female | Male | Size of ITS PCR (bp) | Size of MspI PCR-RFLP (bp) |
|---|---|---|---|---|---|---|---|
| Candida parapsilosis complex | 14 | 10 | 4 | 12 | 2 | 510 or 530 | 510 or 530 |
| Candida albicans complex | 12 | 12 | - | 11 | 1 | 537 | 239, 298 |
| Candida glabrata | 6 | 6 | - | 5 | 1 | 881 | 320, 561 |
| Candida krusei | 5 | 5 | - | 4 | 1 | 510 | 250, 260 |
| Candida tropicalis | 5 | 5 | - | 4 | 1 | 526 | 186, 340 |
| Candida guilliermondii | 3 | 2 | 1 | 3 | - | 607 | 82, 155, 370 |
| Candida famata | 3 | 2 | 1 | 2 | 1 | 639 | 639 |
| Candida kefyr | 2 | 1 | 1 | 2 | - | 720 | 720 |
| Candida species | 1 | - | 1 | 1 | - | ~390 | ~120 , ~270 |
| Total | 51 | 43 | 8 | 44 | 7 |
| Strains (No.) and Antifungal Drugs | S | SDD | R | MIC Range (µg/mL) |
|---|---|---|---|---|
| Candida parapsilosis complex (14) | ||||
| FLC | 13 | - | 1 | 0.5 - 8 |
| ITC | 13 | 1 | - | 0.063 - 0.5 |
| VRC | 14 | - | - | 0.016 - 0.125 |
| POS | 13 | 1 | - | 0.063 - 0.25 |
| Candida albicans complex (12) | ||||
| FLC | 12 | - | - | 0.125 - 2 |
| ITC | 12 | - | - | 0.031 - 0.125 |
| VRC | 12 | - | - | 0.016 - 0.063 |
| POS | 12 | - | - | 0.031 - 0.125 |
| Candida glabrata (6) | ||||
| FLC | - | 5 | 1 | 4 - > 64 |
| ITC | 4 | 2 | - | 0.5 - 1 |
| VRC | 6 | - | - | 0.125 - 0.25 |
| POS | 6 | - | - | 0.25 - 0.5 |
| Candida krusei (5) | ||||
| FLC | - | - | 5 | 16 - 32 |
| ITC | 3 | 1 | 1 | 0.25 - 2 |
| VRC | 5 | - | - | 0.016 - 0.25 |
| POS | 4 | 1 | - | 0.25 - 1 |
| Candida tropicalis (5) | ||||
| FLC | 5 | - | - | 0.125 - 0.5 |
| ITC | 5 | - | - | 0.063 - 0.125 |
| VRC | 5 | - | - | 0.016 - 0.031 |
| POS | 5 | - | - | 0.063 |
| Candida guilliermondii (3) | ||||
| FLC | 3 | - | - | 0.5 - 1 |
| ITC | 3 | - | - | 0.063 - 0.125 |
| VRC | 3 | - | - | 0.016 - 0.031 |
| POS | 3 | - | - | 0.031 - 0.063 |
| Candida famata (3) | ||||
| FLC | 3 | - | - | 0.5 - 1 |
| ITC | 3 | - | - | 0.125 - 0.25 |
| VRC | 3 | - | - | 0.016 |
| POS | 3 | - | - | 0.125 |
| Candida kefyr (2) | ||||
| FLC | 2 | - | - | 0.25 |
| ITC | 2 | - | - | 0.125 |
| VRC | 2 | - | - | 0.016 - 0.031 |
| POS | 2 | - | - | 0.063 - 0.125 |
| Candida species (1) | ||||
| FLC | 1 | - | - | 0.5 |
| ITC | 1 | - | - | 0.125 |
| VRC | 1 | - | - | 0.016 |
| POS | 1 | - | - | 0.125 |
Abbreviations: MIC, minimum inhibitory concentration; S, susceptible; SDD, susceptible dose-dependent; R, resistant.
5. Discussion
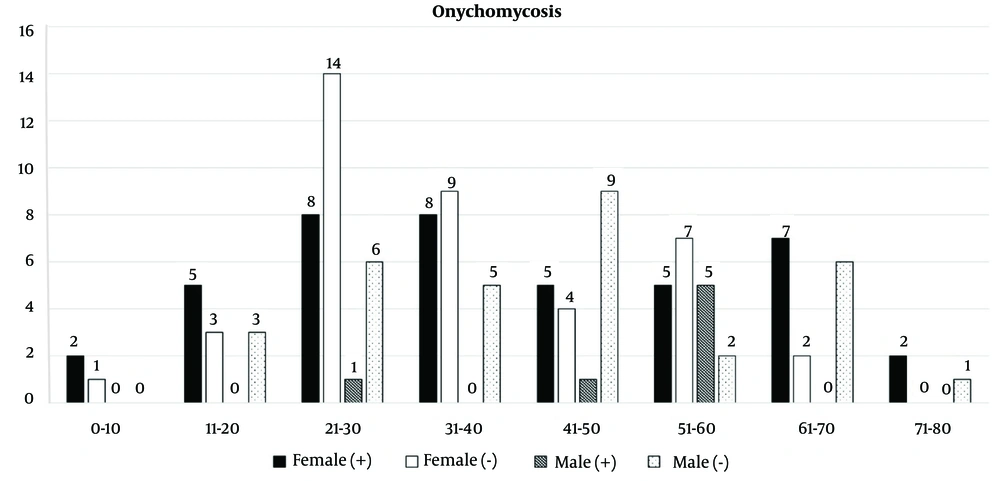
Onychomycosis occurs worldwide and accounts for up to 50% of nail diseases. Its prevalence varies across geographical areas and is influenced by the weather of the region, age, gender, social and occupational factors, the population of immune-deficient patients, and changes in lifestyle and everyday habits (17-19). The present study evaluated the abundance of candidal onychomycosis in different strata of Fasa, Iran, where 51 cases were recorded with candidal onychomycosis from 121 patients over 6 years (2017 - 2023). In this study, 82 (67.8%) of the patients were female, 22 (26.8%) of them were in the range 21 - 30 years, and 39 (32.2%) were male; of this number, 42 (51%) of females had candidal onychomycosis, while only 7 (18%) of males were positive. The abundance of candidal onychomycosis in different age groups showed that patients aged 51 to 60 (19.6%) and 21 to 30 (17.6%) were the most commonly affected, respectively.
Most epidemiological studies in recent years in Iran have reported onychomycosis “between” 35% to 56% of patients (2-7, 20, 21), which is consistent with the present study (36.4%). In all these studies, candidal onychomycosis was more frequent than onychomycosis by molds. In contrast, in the study by Haghani et al. (2019) (22), onychomycosis was diagnosed in 70% of cases, among which 51.1% were caused by non-dermatophyte moulds (NDMs), 35% by yeast and 10.6% by dermatophytes. Also, more positive cases were reported in females than males, more onychomycosis of fingernail than toenail, and most cases of onychomycosis were reported in the age range of 40 - 60 years (2-7, 20, 21), which is consistent with the present study. Also, in most of these studies, C. albicans was the most common cause of onychomycosis, followed by C. parapsilosis (2-7, 20, 21). In contrast, in the present study and the study of Pakshir et al. (2015) in Shiraz (3), the most common species was C. parapsilosis, followed by C. albicans. It is noteworthy that the cities of Fasa and Shiraz are located in the same province (Fars). Therefore, it seems that cases of onychomycosis should be investigated by the mycology laboratory in other cities of Fars province.
In a systematic review study in Iran, Rafat et al. (11) (2019) showed that yeasts were the cause of onychomycosis more than moulds, and females were more affected than males, and, in both genders, the highest infection rate occurred after age 50. In a worldwide review study, Piraccini and Alessandrini reported that onychomycosis was mainly caused by anthropophilic dermatophytes, in particular by Trichophyton rubrum and T. interdigitale, and yeasts such as C. albicans and C. parapsilosis; molds such as Aspergillus spp., represented the second cause of onychomycosis (1). In the study in Greece by Gregoriou et al. (17), in 27.99% of diagnosed onychomycosis cases, men were infected more often than women, and toenails were infected more than fingernails in both sexes. Also, Bodman and Krishnamurthy (2022) reported that onychomycosis of the toenail was much more prevalent (23).
In a retrospective study in Brazil, Arrua et al. showed that onychomycosis most affected women, which occurred mainly in adults (24). The toenails were the favorite yeast targets, and the prevalent yeasts were C. tropicalis and C. krusei. In another study in Egypt, Bedaiwy et al. showed that candidal onychomycosis was the 5th most common clinical type, C. tropicalis was the most prevalent causative species, fingernails were affected more frequently than toenails, and the infection was more common among females aged 41 to 50 years (mostly housewives) (18).
Today, with the emergence of resistant strains due to using azoles to prevent fungal infections, the in vitro antifungal susceptibility testing of Candida species for the proper treatment of patients is very important. However, in our study, the results of in vitro antifungal susceptibility testing of 51 Candida species revealed that all isolates (100%) of C. albicans complex, C. tropicalis, C. famata, C. guilliermondii, C. kefyr, and one Candida species were susceptible to four triazoles. Only one C. parapsilosis complex was resistant to FLC and SDD to ITC and POS. However, in some studies, C. albicans is demonstrated to be resistant to some antifungal drugs, especially fluconazole, which is widely used to prevent fungal infections (25-28). Different studies showed in vitro antifungal activity against various species of Candida in Iran. For instance, Aslani et al. in 2019 and 2021, reported that some of C. albicans isolates were resistant to FLC (4.3%, 3.5%), ITC (6.5%, 4.7%), VRC (2.1%, 1.1%) and POS (6.5%, 4.7%), and C. non- albicans were resistant to FLC (43.4%, 21.7%), ITC (12.9%, 25%) and POS (1.5%, 12.5%) (25, 26).
A study by Shokohi et al. in 2016 showed that 9.1 %, 11.3%, and 9.1 % of Candida albicans isolates were resistant to FLC, ITC, and VRC, respectively (28). Pakshir et al. showed only one isolate (2%) of C. parapsilosis was resistant to FLC, and two isolates (4%) were resistant to VRC. In contrast, 43% of C. albicans isolates and (54%) of C. tropicalis were resistant to VRC (3). Fortunately, in this study, most Candida isolates were susceptible to antifungal drugs and had low resistance against triazoles. It is noteworthy that in the present study, most referred women were housewives. In fact, it can be argued that women place more importance on beauty than men. At the same time, at the community level, there may be men suffering from onychomycosis but indifferent to it. Undoubtedly, onychomycosis is often regarded as a trivial cosmetic issue. However, its effect on one’s quality of life may be underestimated as it can cause significant pain, affecting full mobility and activities. Patients working in moist environments such as dishwashing areas should be encouraged to wear gloves because humidity and maceration can cause onychomycosis by Candida species.
5.1. Conclusions
The prevalence of fingernail onychomycosis in Iranian housewives has increased in recent decades. In summary, this study showed that identifying Candida species by molecular methods such as PCR-RFLP and in vitro antifungal susceptibility tests can aid physicians in choosing effective drugs for treating onychomycosis. Therefore, it is necessary to have specialized medical mycology laboratories in every city.