1. Background
The prevalence of invasive fungal infections has been increasing in immunocompromised patients (1). Invasive pulmonary aspergillosis (IPA) is a severe and lethal fungal disease mainly caused by fungi of the genus Aspergillus, especially Aspergillus fumigatus (2). It mainly occurs in long-term neutropenic patients, recipients of solid organ or allogeneic hematopoietic cell transplants, blood malignancy patients, those using high-dose corticosteroids, non-neutropenic patients with chronic obstructive pulmonary disease, and patients with severe genetic immune deficiencies (2, 3). In late 2019, with the emergence of COVID-19, secondary respiratory microbial pulmonary co-infection (including pulmonary aspergillosis) was progressively recognized in hospitalized COVID-19 patients (4). Similarly, during the early period of the COVID-19 pandemic, COVID-19-associated PA (CAPA) was described (5).
COVID-19-associated pulmonary aspergillosis was reported in up to 4% of individuals with COVID-19 from China (6). In other studies, the reported incidence of CAPA differs from 3 to 39% (7-9). However, little is known about its exact prevalence, clinical significance, or associated outcomes in Iran. The availability of local data about CAPA can help in solving many challenges of early diagnosis and treatment of IPA during the COVID-19 outbreak. During the COVID-19 epidemic, CAPA was identified as a distinct entity (4). A severe COVID-19 results in pulmonary epithelial failure and a hyperinflammatory response or cytokine storm that often requires the prescription of corticosteroids to patients. Individuals with severe COVID-19 are prone to IPA, which can lead to death (10). The incidence range of CAPA was reported as 3 - 39% (7-9). Early diagnosis followed by prompt administration of appropriate antifungal therapy is crucial for decreasing the mortality rate of IPA patients (2, 11).
The gold standard for the detection of CAPA is the culture and direct smear of tissue and bronchoalveolar lavage fluid. However, doing this is very challenging in patients with COVID-19. Biopsy may not be possible due to severe thrombocytopenia (11-13). In addition, due to the risk of COVID-19 transmission, bronchoscopies are not performed in these patients (10-12). For these reasons, we used serum galactomannan (GM) (11, 12), which is a major polysaccharide component of Aspergillus cell walls (1). This heat-stable soluble antigen is released (1) by fungal hyphae in the serum and other body fluids and subsequently can be detected by sandwich-enzyme immunoassay (3, 11). Other researchers have investigated the role of vitamin D in controlling aspergillosis and COVID-19 separately. In addition, it has been found that vitamin D deficiency plays a role in respiratory infections (14, 15).
2. Objectives
There is ambiguity about the prevalence of CAPA and its associated factors. To contribute to a better management of CAPA, our study aimed to evaluate the serum levels of GM antigen among people with COVID-19 to clarify the aspects of CAPA, focusing on its prevalence, risk factors, treatment, and outcome for the first time in southwestern Iran. The second objective was to determine the role of vitamin D in patients with CAPA.
3. Methods
3.1. Study Design and Participants
This study is a cross-sectional research performed during the third to fifth waves of COVID-19 in the intensive care unit (ICU) of 6 tertiary care hospitals in Ahvaz City (Razi, Sina, Imam Khomeini, Amir-Al-Momenin, Golestan, Shahid Baghaei) between September 2020 and November 2021 (a period of 11 months). Eventually, 257 patients with COVID-19 who were admitted to ICU entered this work. This study used the CAPA classification according to the recent EORTC/MSGERC (European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium) 2020 criteria (16).
To diagnose IPA in patients with COVID-19, we just used serum. Hence, based on the present criteria, none of the studied cases could be considered a proven IPA. However, a positive result could be considered a probable IPA. The sociodemographic details, laboratory findings, treatment, and outcomes of the patients were extracted from the medical documents available on the hospital information systems (HIS). Unfortunately, due to some predictable shortcomings and problems during the pandemic, we only had access to the information on 51 CAPA cases. The inclusion criteria of this research were patients admitted to the ICU who had definite confirmation of laboratory diagnosis for COVID-19 by a molecular test (real-time polymerase chain reaction [PCR]) and were suspected of IPA based on clinical and/or radiological evidence of symptoms.
3.2. Serum Galactomannan Test
The serum was taken from 257 patients suspected of CAPA upon admission and stored at -20°C until tested for GM. Aspergillus GM assay kit with lot number 210605-HW was used for the quantitative detection of Aspergillus GM antigen in serum samples, offering a diagnostic reference based on competitive enzyme-linked immunosorbent assay (ELISA) for Aspergillus infection according to the manufacturer’s instructions (bioactive diagnostic GmbH, Homburg, Germany). The cutoff value ≥ 0.5 was considered a positive result. CAPA was defined according to the revised EORTC/MSGERC 2020 criteria. Patients with clinical findings, abnormal results in lung computed tomography (CT) scans, and positive serum GM antigen were classified as probable IPA (17).
3.3. Vitamin D Assay Test
The 25-Hydroxy Vitamin D (25(OH)D) assay was performed according to the manufacturer’s instructions (manufacturing company, pioneers of Isatis assay research) based on the competitive saturated ELISA. The threshold of 10 ng/mL is considered equivalent to vitamin D deficiency. The range of 10 - 29 ng/mL is the insufficient range of vitamin D, and the level of at least 30 ng/mL 25(OH) D is considered to be sufficient. The vitamin D level was measured for the CAPA population.
3.4. Statistical Analysis
The statistical analysis was performed using SPSS version 23 (SPSS Inc, Chicago, IL, USA). The data were assessed by descriptive statistics (frequency, percentage, mean, SD). The Shapiro-Wilk test was used to check normality. The survival status and GM values between the groups were compared using Mann-Whitney and Kruskal Wallis tests. The correlations between the survival status/GM index and the quantitative variables were investigated using Spearman's rank test. P-values less than 0.05 were considered statistically significant.
4. Results
All the patients in this research were COVID-19 positive. Due to the existing treatment protocol, all of them had a history of receiving remdesivir with a range of 400 - 1200 (mg) and a mean (SD) of 6.86 (2.28). All the patients had fever and chills. The average age was 53.24 ± 13.79 years (range: 29 - 82 years), and the mean ICU stay was 24.55 ± 20.14 days. Serum GM results (cutoff value of ≥ 0.5) were detected in 114 (44.35%) out of 257 COVID-19 patients. These included 63 (47.73%) males and 51 (41.8%) females, with a female-to-male ratio of 1: 1.23. According to the national guidelines, the course of COVID-19 infection has 4 stages (from 0 to 3). The second stage (respiratory phase) includes 2 groups, namely, moderate and severe, while the third stage is known as the inflammatory or critical phase. Nine patients in our survey were in the critical phase (SpO2 ≤ 88% despite non-invasive oxygen therapy treatments) under mechanical ventilation. Twenty patients were in the severe respiratory phase (RR > 30; SpO2 < 90%; PaO2/FiO2 ≤ 300 mm Hg, involvement of more than 50% of the lung based on CT scans), and 22 other patients were in the moderate breathing phase (SpO2 between 90 - 93%). All patients who were followed up in this study received either high-flow nasal oxygen therapy or non-invasive ventilation.
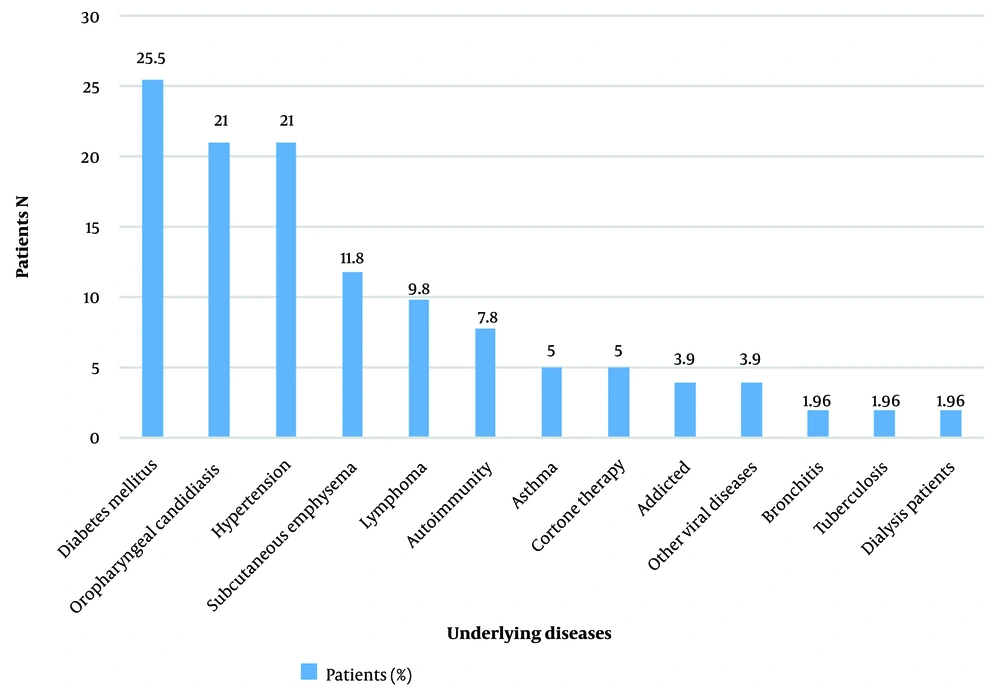
In Table 1, the distributions of the patients with CAPA during COVID-19 waves and gender are summarized. Patients with CAPA were more frequently diagnosed during Delta and Alpha waves. Generally, 52.9% of the patients with CAPA had an underlying disease, with diabetes mellitus (25.5%) as the most common, followed by oropharyngeal candidiasis (21%), hypertension (21.6%), subcutaneous emphysema (11.8%), and lymphoma (9.8%), respectively (Figure 1).
| COVID-19 Wave; Gender | No. (%) | Third Wave (Beta Strain); Winter 2021 | Fourth Wave (Alpha Strain); Spring 2021 | Fifth Wave (Delta Strain); Summer and Autumn 2021 |
|---|---|---|---|---|
| Female | 122 (47.47) | 24 | 39 | 59 |
| Men | 135 (52.53) | 26 | 68 | 41 |
| Female with pos GM | 51 (41.8) | 4 | 14 | 33 |
| Men with pos GM | 63 (47.73) | 5 | 37 | 21 |
| Total Pos | 114 (44.36) | 9 | 51 | 54 |
| Total Neg | 143 (55.64) | 41 | 56 | 46 |
| Total | 257 | 50 (19.45) | 107 (41.65) | 100 (38.9) |
Gender Distribution of Patients with CAPA During the Third to Fifth COVID-19 Waves
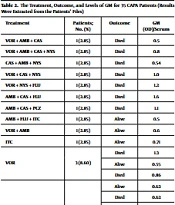
Thirty-five CAPA patients (68.63%) received antifungal agents after the GM test was positive. The GM of these 35 patients ranged from 0.5 to 4.6. In 6 out of 18, 5 out of 12, and 2 out of 2 subjects who had respectively received voriconazole, amphotericin B, and itraconazole (either alone or with any other antifungal agents), a successful outcome was observed. The results showed that treatment failed in all 7 (100%) patients who had only received caspofungin. The fates of these 35 patients and the details of the antifungal agents used for them are summarized in Table 2. The mortality rates among all patients with CAPA and individuals under antifungal treatment were 66.7% and 68.57.1%, respectively.
| Treatment | Patients; No. (%) | Outcome | GM (OD)/Serum |
|---|---|---|---|
| VOR + AMB + CAS | 1 (2.85) | Died | 0.5 |
| VOR + AMB + CAS + NYS | 1 (2.85) | Died | 0.8 |
| CAS + AMB + NYS | 1 (2.85) | Died | 0.54 |
| VOR + CAS + NYS | 1 (2.85) | Died | 1.0 |
| VOR + NYS + FLU | 1 (2.85) | Died | 1.2 |
| AMB + CAS + FLU | 1 (2.85) | Died | 1.6 |
| AMB + CAS + PCZ | 1 (2.85) | Died | 1.1 |
| AMB + FLU + ITC | 1 (2.85) | Alive | 0.5 |
| VOR + AMB | 1 (2.85) | Alive | 0.6 |
| ITC | 1 (2.85) | Alive | 0.71 |
| VOR | 3 (8.60) | Died | 1.3 |
| Alive | 0.55 | ||
| Died | 0.86 | ||
| VOR + FLU | 5 (14.3) | Alive | 0.62 |
| Died | 0.62 | ||
| Died | 0.64 | ||
| Alive | 0.60 | ||
| Died | 4.6 | ||
| VOR+ NYS | 5 (14.3) | Died | 0.66 |
| Died | 0.65 | ||
| Died | 1.68 | ||
| Alive | 0.82 | ||
| Alive | 1.25 | ||
| AMB + NYS + FLU | 5 (14.3) | Alive | 0.5 |
| Alive | 0.7 | ||
| Alive | 0.6 | ||
| Died | 0.63 | ||
| Died | 0.51 | ||
| CAS | 7 (20.0) | Died | 1.0 |
| Died | 2.99 | ||
| Died | 0.6 | ||
| Died | 0.5 | ||
| Died | 0.5 | ||
| Died | 0.5 | ||
| Died | 0.61 | ||
| Total | 35 (100) | Alive = 11 (31.43%) | (0.5 - 4.6) |
| Died = 24 (68.57%) |
The Treatment, Outcome, and Levels of GM for 35 CAPA Patients (Results Were Extracted from the Patients’ Files)
In this study, we examined the clinical and epidemiological characteristics of 51 cases of CAPA. The analysis included both quantitative and qualitative variables and was conducted on cases that were available in the HIS. Among these cases, we compared the characteristics of those who survived with those who did not (Tables 3 and 4). In the surviving individuals, CAPA had a statistically significant relation with age (r = 0.520; P = 0.032), blood sugar (BS; r = 0.497; P = .043), diagnosis of hyperglycemia (elevated fasting BS > 125 mg/dL) during hospitalization (P = 0.039), and diabetes mellitus (P = 0.039). In deceased patients, CAPA had a significant association with age (r = 0.503; P = 0.002).
There was no significant relationship between other variables (quantitative and qualitative variables) in deceased and surviving patients (P > 0.05). Furthermore, when assessing the level of vitamin D, our study revealed that the CAPA population exhibited 72.5% insufficiency and 13.7% deficiency, according to the established criteria for vitamin D levels. We calculated the correlation of the GM index and quantitative variables, as noted in Table 3, and did not find a statistically significant correlation with those variables except age (r = 0.519; P < 0.0001). In Table 4, among the various parameters with the GM index, the P-value > 0.05. Therefore, no significant relationship was observed among the CAPA patients with qualitative variables.
| Variables | Normal Range | Patient Range | Surviving (n = 17) | Deceased (n = 34) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | r | P-Value a | Mean ± SD | r | P-Value a | |||
| Age, y | - | 29 - 82 | 51.35 ± 3.24 | 0.520 | 0.032 a | 54.18 ± 2.42 | 0.503 | 0.002 a |
| GM duration, db | - | 1 - 36 | 8.94 ± 2.18 | 0.254 | 0.326 | 14.00 ± 1.65 | -0.059 | 0.738 |
| BS, mg/dL | 75 - 115 | 70 - 420 | 206.00 ± 19.62 | 0.497 | 0.043 | 252.62 ± 13.32 | -0.133 | 0.453 |
| CRP, mg/L | < 10 | 22 - 129 | 53.06 ± 5.02 | 0.166 | 0.524 | 67.71± 4.99 | 0.067 | 0.706 |
| Vitamin D, ng/mL | 30 - 100 | 7.5 - 58.6 | 20.01 ± 2.35 | -0.071 | 0.785 | 20.17 ± 2.22 | 0.231 | 0.188 |
| Hospitalization duration, d | - | 5 - 135 | 20.47± 3.67 | 0.109 | 0.676 | 26.59 ± 3.80 | -0.121 | 0.496 |
| IL-6, pg/mL | ≤0.5 | 8 - 47 | 17.96 ± 1.52 | 0.121 | 0.644 | 27.37 ± 1.56 | -0.161 | 0.362 |
| Procalcitonin, µg/L | ≤0.01 | 0.01 - 9.8 | 6.41 ± 0.46 | 0.114 | 0.663 | 7.09 ± 0.42 | -0.162 | 0.359 |
| Respiratory rate, breaths/min | 12-20 | 13 - 45 | 0.82 ± 0.59 | -0.154 | 0.555 | 0.57 ± 0.17 | -0.302 | 0.083 |
| Blood pressure, mmHg | 120/80 | 50/95 - 130/80 | 24.94 ± 1.61 | 0.130 | 0.620 | 29.62 ± 1.44 | 0.096 | 0.589 |
| Hospitalization duration, d | - | 8 - 78 | 81.12 ± 4.63 | 0.131 | 0.617 | 81.31 ± 1.96 | -0.032 | 0.857 |
| Blood oxygen level, % | 95/98 | 50/55 - 100/92 | 88.85 ± 2.87 | -0.146 | 0.576 | 85.95 ± 1.64 | 0.164 | 0.355 |
| Temperature, °C | 36.5 - 37.2 | 37.5 - 40 | 38.01 ± 0.11 | 0.210 | 0.418 | 38.22 ± 0.09 | -0.165 | |
Demographic, Clinical, and Laboratory Characteristics (Quantitative Variables) of COVID-19 Patients with Pulmonary Aspergillosis
| Variables | Surviving | Deceased | ||||
|---|---|---|---|---|---|---|
| No. (%) | Mean ± SD | P-Value a | No. (%) | Mean ± SD | P-Value a | |
| Gender | 0.106 | 0.130 | ||||
| Female | 11 (64.7) | 0.12 ± 0.60 | 22 (64.7) | 1.06 ± 0.87 | ||
| Male | 6 (35.3) | 0.26 ± 0.77 | 12 (35.3) | 0.90 ± 0.72 | ||
| Underlying disease | 0.735 | 0.369 | ||||
| Negative | 8 (47.1) | 0.62 ± 0.11 | 16 (47.1) | 0.99 ±1.01 | ||
| Positive | 9 (52.9) | 0.70 ±0.24 | 18 (52.9) | 1.01 ± 0.63 | ||
| Diabetes mellitus | 0.039 | 0.776 | ||||
| Negative | 12 (70.6) | 0.60 ± 0.10 | 26 (76.47) | 1.04 ± 0.90 | ||
| Positive | 5 (29.4) | 0.82 ± 0.26 | 8 (23.53) | 0.89 ± 0.44 | ||
| Suspected of fungal infection | 0.920 | 0.003 | ||||
| Negative | 11 (64.7) | 0.68 ± 0.22 | 28 (82.35) | 0.78 ± 0.35 | ||
| Positive | 6 (35.3) | 0.63 ± 0.12 | 6 (17.65) | 2.02 ± 1.49 | ||
| Pulmonary cavity | 0.205 | 0.509 | ||||
| Negative | 14 (82.35) | 0.63 ± 0.12 | 27 (79.4) | 0.97 ± 0.82 | ||
| Positive | 3 (17.65) | 0.84 ± 0.37 | 7 (20.6) | 1.12 ± 0.86 | ||
| Hyperglycemia during hospitalization | 0.039 | 0.776 | ||||
| Negative | 5 (29.4) | 0.57 ± 0.10 | 8 (23.53) | 0.86 ± 0.44 | ||
| Positive | 12 (70.6) | 0.82 ± 0.26 | 26 (76.47) | 1.04 ± 0.90 | ||
Frequency and Distribution of the Underlying Conditions (Qualitative Variables) in 51 Patients with CAPA and Their Survival Status
5. Discussion
COVID-19-associated pulmonary aspergillosis is recognized as a major complication of critically ill COVID-19 patients (18, 19). The current survey estimated the prevalence of CAPA to be 44.35% among all COVID-19 patients. Nevertheless, in different investigations, there have been different prevalence rates of CAPA, ranging from 1.0 to 47.4% among COVID-19 patients admitted to ICU with or without mechanical ventilation (17, 20). The discrepancies between studies may reflect the wide variation of classification criteria and CAPA definitions (especially early in the COVID-19 pandemic), type of test (antigen detected or direct smear), sample type (sputum, broncho-alveolar lavage [BAL], bronchial brush), national treatment guidelines, environmental factors, patient populations, local epidemiology, duration of study, and investigation in different waves with different severities.
The mortality rates among all patients and individuals under antifungal treatment were 66.7% and 68.57.1%, respectively, which is similar to previous studies that reported mortality rates exceeding 60% despite appropriate treatment (18, 21). The reason for most of the deaths in the fourth and fifth waves was the more contagious nature of the Delta variant compared to that of the original Wuhan SARS-CoV-2 strain (22), as well as the hyperglycemia caused by the administration of some drugs such as remdesivir (23). These results reflect the life-threatening entity of IPA and emphasize early detection and prompt administration of appropriate antifungal treatment. Consistent with our findings, recent studies have also reported an association between older age (18, 24, 25) and the risk of contracting various diseases, including IPA. This association may be attributed to the changes that occur with aging, which can affect the functioning of the immune system and make older individuals more susceptible to developing such diseases (26). Despite our prospects, no statistical difference was found in the gender variable. This result is consistent with studies carried out by Chong et al. (24) but is contrary to other findings indicating that gender is associated with hospital mortality among CAPA patients (18).
Our analysis of variables related to BS disorders demonstrated the importance and impact of hyperglycemia on IPA. In addition, it was found that the mean BS levels of the deceased group were higher than those of the surviving group. Consistent with our investigation, Ghanaat and Tayek also identified diabetes as an important risk factor for the development of IPA (27). More than 25% of our patients had a history of diabetes as an underlying disease, and hyperglycemia occurred during hospitalization in 38 cases (74.5%). Some studies have shown that COVID-19 itself leads to disturbances in the regulation of BS. On the other hand, people with hyperglycemia infected with COVID-19 have a higher rate of severe pneumonia and mortality compared with non-hyperglycemia subjects (28). Therefore, BS monitoring greatly contributes to the prognosis and management of CAPA patients. Screening for diabetes is recommended in individuals who have recovered from even mild COVID-19 (29).
Twelve of the 18 CAPA patients who died had received voriconazole as a treatment choice for IPA. The failure of treatment with voriconazole in our patients may be attributed to these drug-drug interactions with remdesivir (10). As noted in other studies, itraconazole is an alternative drug for the treatment of CAPA and has antiviral activities, whereas echinocandins have little impact on IPA (10). Perhaps for this reason, in this study, the treatment of the 2 patients receiving itraconazole was successful, whereas the treatment of the patients receiving caspofungin failed. Generally, the response to an antifungal agent is influenced by the underlying disease and the stage of CAPA. Finally, more studies are needed to obtain a paradigm for the treatment of CAPA. Our analyses did not show a statistically significant relationship between vitamin D and GM or survival status. However, what is noteworthy is that 86.27% of our patients had insufficient vitamin D levels (range: 26.0 - 7.5 ng/mL). It is suggested that patients suspected of IPA be evaluated in terms of vitamin D levels and that vitamin D deficiency be measured as a risk factor for IPA in future studies.
The main limitation of our study is that the detailed information of all participants was not available due to the irregularities caused by health care system overload during the COVID-19 pandemic, and only the data of 51 CAPA-positive patients were analyzed. As a result, it was not possible to perform further statistical analyses between the CAPA group and COVID-19 patients with negative GM. Another point was that due to the cross-sectional design of this survey, we could not follow the patients after discharge. In spite of these limitations, we believe that our results are of interest and can inspire further studies with larger numbers, considering that the prevalence of CAPA and the analysis of risk factors related to the GM index are published for the first time from the southwest of Iran. Another strong point of this study is that information bias was unlikely due to the use of original data collected in the HIS.
5.1. Conclusions
This research showed that IPA was a diagnostic-therapeutic challenge in patients with COVID-19, as well as other similar critical populations such as those with severe influenza and other respiratory viral infections. Furthermore, our results demonstrated that age and chronic or temporary hyperglycemia were the most important factors in relation to the survival status of patients.