1. Background
Vulvovaginal candidiasis (VVC) is a prevalent opportunistic mucosal infection that may affect a significant majority of women, with approximately 75% experiencing it during their lifetime (1). Within this population, 5 - 10% endure the challenges of recurrent VVC (RVVC), characterized by more than three episodes of infection per year (2, 3). The principal culprit behind vaginal fungal infections is Candida albicans, closely followed by the non-albicans Candida (NAC) strain, C. glabrata (4). Both VVC and RVVC present distressing symptoms, including pain, itching, burning, dyspareunia, and abnormal vulvar discharge. However, RVVC patients often exhibit greater severity and clinical manifestations (5-7). Despite effective antifungal therapy, RVVC patients commonly face a recurrence of clinical manifestations (1), and the factors contributing to this recurrence remain inadequately understood (7). The quest to understand the high prevalence of RVVC has led to a spotlight on host genetic factors and fungal virulence, as the precise causes remain elusive (2).
Unlike systemic candidiasis patients, RVVC patients may not possess significant immunodeficiency, and C. albicans is normal flora in vaginal tissue (6). Minor alterations in the vaginal mucosa and its immunity are implicated in RVVC (4), suggesting that local immunity may play a more pivotal role compared to the systemic counterpart (3). The transition of Candida spp. to the hyphal form within the mucosa intensifies local inflammatory reactions, providing insights into the dynamic nature of mucosal candidiasis (6). Examination of the immunological aspects of RVVC has revealed insights into its substantial impact on the quality of life and the associated healthcare costs (8). Moreover, the presence of distinct host immune responses in RVVC underscores the intricate involvement of the immune system in this condition (9).
Mucosal Candida infections are widely dependent on cell-mediated immunity (CMI), with a heightened incidence observed in conditions such as acquired immunodeficiency syndrome (AIDS), post-transplantation, and corticosteroid therapy, thus reinforcing the hypothesis of the role of CMI in infection (10). The pivotal role of T helper (Th) type 1 and Th2 responses in dictating disease outcomes is underscored by the delicate balance required between these cytokine responses (10). The CMI by Th1 responses is presumed to have a resistance effect, while Th2 responses lead to mucosal candidiasis (3).
Several studies have demonstrated that transforming growth factor-beta (TGF-β) emerges as a potent down-regulatory cytokine crucial for maintaining vaginal mucosal homeostasis (11, 12). Additionally, elevated levels of TGF-β isoforms are associated with the induction of Th17 responses, unleashing pro-inflammatory cells that secrete interleukin (IL)-17 and confer immunity in the vagina, particularly against Candida (13, 14) and reduce the consequences of VVC and RVVC (11). Ultimately, RVVC emerges as a complex disorder impacting microbial, immune, and sexual health in a considerable segment of adult females (15). Grasping the immunopathology of RVVC, with a particular focus on the role of TGF-β, is essential for crafting effective immunotherapeutic strategies and tailoring personalized treatment approaches (11, 16).
2. Objectives
As a result, this study aimed to evaluate the expression levels of the TGF-β gene in RVVC patients compared to the control group in order to possibly establish an index for diagnosing prognosis and perhaps identify a potential target for controlling the symptoms of the disease.
3. Methods
3.1. Patients and Samples
This descriptive study aimed to assess 750 symptomatic patients suspected of having Candida vaginitis who were referred to gynecology and obstetrics clinics in Bushehr, a city in southern Iran, over a two-year period. To be considered for RVVC, patients had to have experienced at least three episodes of VVC in the previous 12 months, confirmed by clinical presentation and microscopic examination of samples.
The study included 124 patients with RVVC and 225 age-matched healthy individuals who had no underlying diseases as the control group. The methods for sampling, direct diagnosis, culturing, and molecular identification of Candida species in RVVC patients have been detailed in our previous study (17). The presence of other concurrent infections and causes of vaginitis was ruled out by a gynecologist. Notably, the patients were not undergoing any specific antifungal treatments at the time of sampling. Control group members were selected based on negative mycological tests, and any history of vaginal infection or autoimmune diseases served as exclusion criteria. Serum from all specimens was immediately separated after blood collection, aliquoted into 1.5 mL cryotubes, and stored at -80°C for subsequent analysis.
3.2. Gene Expression Analysis Using Quantitative Real-time Polymerase Chain Reaction
For RNA isolation, RNA from whole serum was extracted using the High Pure RNA Isolation Kit (Roche, Mannheim, Germany). To synthesize complementary DNA (cDNA) strands, the Thermo Scientific Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., USA) was employed. The purity and concentration of the cDNA were determined by measuring the 260/280 nm and 260/230 nm ratios with a NanoDrop DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA). Subsequently, all qualified cDNA was stored at -20°C. Primers for the GAPDH gene were manually designed, while primers for the TGF-β gene were acquired from the study by Jaberipour et al. (18) and synthesized by Bioneer Company (Table 1).
Quantitative real-time polymerase chain reaction (QRT-PCR) was conducted using SYBR Green Master Mix (Yektatajhiz CO., Iran) on an ABI StepOnePlus RT-PCR system (Applied Biosystems, USA). Each reaction, with a final volume of 15 µL, included 7.5 µL of SYBR Green Master Mix (2X), 1 µL of each forward and reverse primer (10 pmol/µL), 1 µL of cDNA, 0.3 µL of ROX reference dye, and 5.2 µL of RNase-free water. The amplification protocol started with an initial denaturation cycle at 95°C for 10 minutes, followed by 40 amplification cycles at 95°C for 15 seconds, and a combined annealing and extension phase at 60°C for 30 seconds. Additionally, a non-template control (NTC) was employed in endpoint PCR experiments to confirm the absence of contamination in the real-time PCR assays. The relative quantification of target gene expression was calculated using the 2-ΔΔCt method (19), with cycle threshold (Ct) values normalized against GAPDH as the internal control.
| Gene Name | Primer | Nucleotide Sequences (5′-3′) |
|---|---|---|
| TGF-β | TGF-β FTGF-β R | TGGTTGAGCCGTGGAGGGGACTCGGCGGCCGGTAGTGAAG |
| GAPDH | GAPDH FGAPDH R | AAGGTGGTGAAGCAGGCGAGCGTCAAAGGTGGAGGAG |
The Sequences of Used Primers for Real-time Polymerase Chain Reaction Analysis
3.3. Statistical Analysis
Data analysis was performed using IBM SPSS software, version 26 (SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test assessed the normality of data distribution. To compare gene expression differences between healthy controls and patients, the Mann-Whitney U test was utilized. Data visualization was achieved with GraphPad Prism version 9 (GraphPad Software, Inc., San Diego, CA, USA). A P-value of less than 0.05 was deemed statistically significant, and results were presented as mean ± standard error of the mean (SEM).
4. Results
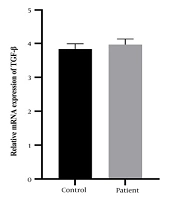
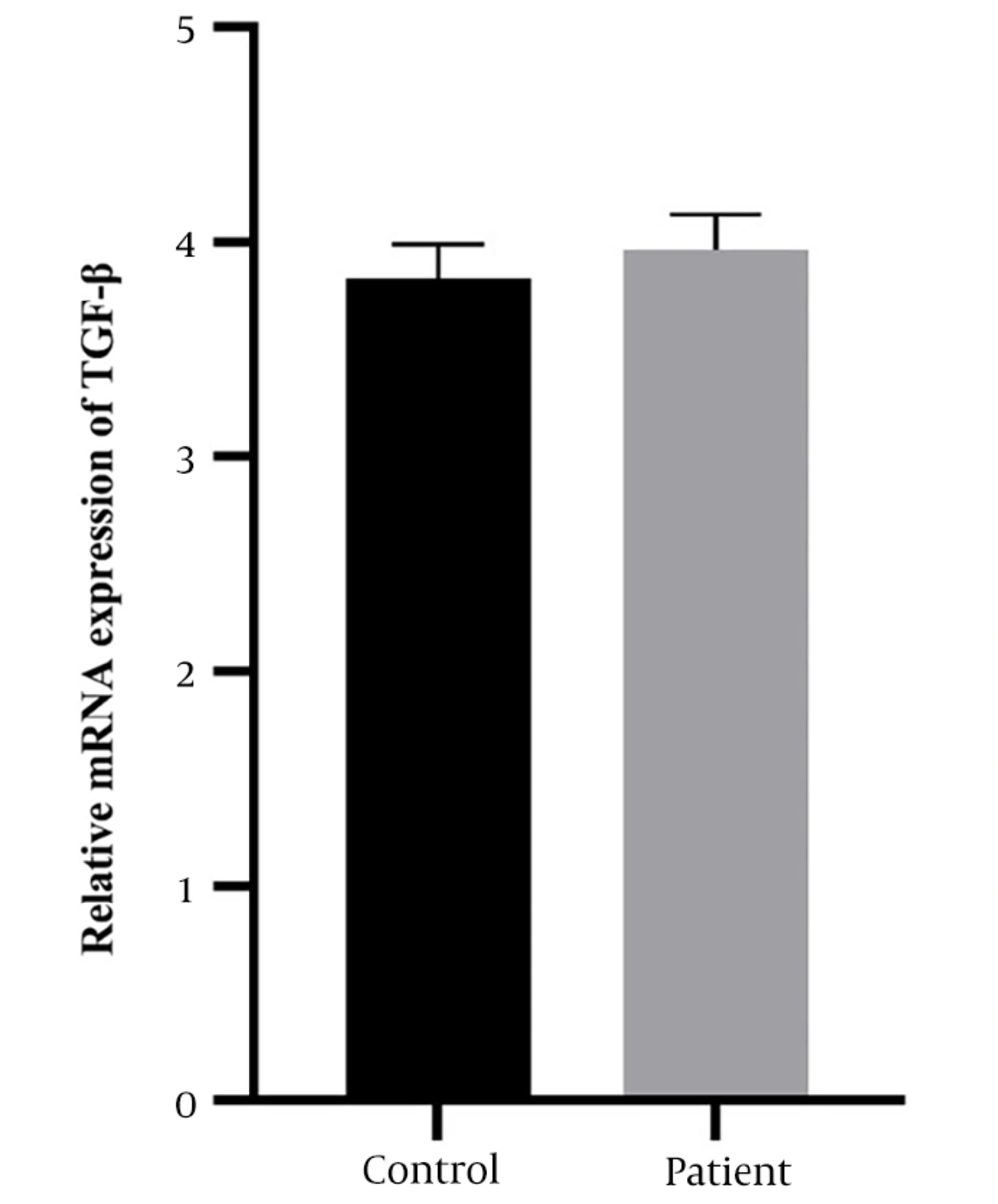
The case-control study included 124 diagnosed patients with RVVC and 225 individuals in the control group. As shown in Figure 1, the results of TGF-β expression illustrate that comparisons of mRNA levels showed an increase in serum TGF-β expression levels in the patient group (3.96 ± 0.16) compared to the control group (3.83 ± 0.15). Therefore, the serum levels of TGF-β did not show a significant difference between the patient and the control group (P = 0.2639).
5. Discussion
Candidal vulvovaginitis is recognized as one of the most common and significant infectious complications in women throughout their lives, becoming even more complex in its recurrent form, RVVC (1, 4). Minor changes in mucosal integrity and local immunity can precipitate disease when C. albicans colonizes and proliferates in the vaginal tissue (6, 20), highlighting the importance of local immunity in the disease's prevalence (3). Cell-mediated immunity and its secreted cytokines are crucial in the defense against mucosal Candida infections, where even minor alterations in cytokine response balance can lead to disease (10). Some studies have identified TGF-β as a cytokine that down-regulates immune response in the vaginal mucosa, thereby mitigating disease outcomes (11, 12, 16), whereas others present opposing findings (21, 22).
Notably, Wozniak et al. found elevated levels of TGF-β in humans and mice subjected to infection (22). Recent research has underscored the role of TGF-β in the immune response and regulation during fungal infections. In murine models, Candida infection has been shown to induce the upregulation of TGF-β, especially in the liver and lungs. Moreover, TGF-β, along with other immunoregulatory factors, indicates immunoregulation at the vaginal mucosa, limiting the efficacy of the Th1-type protective immune response. Neither intravaginal nor systemic Th1-type cytokines, anti-IL-10, anti-TGF-β antibodies, nor adenoviruses encoding Th1-type cytokines have proven protective against VVC. The expression of adenovirus vectors encoding interferon-gamma (IFN-γ) showed much higher efficiency in vitro than in vivo.
An additional study on Candida vaginitis, conducted by Fidel Jr. et al. (as cited by Santoni et al.) (23), underscores the importance of host defense mechanisms in determining resistance or susceptibility to infection. Protection is linked to a non-inflammatory response, while an inflammatory response mediated by polymorphonuclear neutrophils (PMNs) tends to result in vaginitis or mucosal infections. Vaginal lymphocytes (VL), including clusters of differentiation (CD) such as CD4 and CD8 T cells or CD3 and CD5 B cells, have demonstrated protective roles in the vaginas of both naive and immune rats (17). Animal models receiving total VL or CD3 T cells exhibited increased fungal clearance from their vaginas. CD3 CD5 B cells were effective in rats, but compared to immune T cells, they had a lesser impact on reducing Candida colony-forming units (CFU).
Among T cell populations, CD4 T cells were significantly more effective than CD8 T cells. Furthermore, histological analyses of rat vaginal tissues revealed lymphocyte accumulation in the mucosal epithelium. This research reinforces the hypothesis of local T cell and Th-type immunity in protection. In our study, we aimed to measure TGF-β gene expression in RVVC patients compared to age-matched healthy participants without underlying disease as a control group. Despite an increase in TGF-β levels, it was not statistically significant (P = 0.2538).
Another study by Dadak et al. (24) showed elevated TGF-β1 levels in patients with chronic mucocutaneous candidiasis (CMC) compared to a control group. However, no significant differences were observed in serum TGF-β1 levels between the groups, with patient levels at (685 pg/mL) and controls as mean ± standard deviation at (578.4 pg/mL ± 573). They also noted that serum TGF-β1 levels were significantly higher than plasma levels. The findings of this study, regarding the TGF-β1 isoform examined, a modest increase in patients compared to controls, and the nonsignificance of this difference, were very similar to our results. However, the study's patient, despite having a recurrent vaginal candidal infection, was diagnosed with CMC (not RVVC), a more severe form of candidal infection. Additionally, in this study, TGF-β1 serum levels were measured using enzyme-linked immunosorbent assay (ELISA), whereas our study evaluated gene expression levels. Steele and Fidel Jr (25) assessed the production of cytokines and chemokines in oral and vaginal epithelial cells in response to C. albicans colonization.
Primitive cytokines, such as IL-1 and tumor necrosis factor (TNF), were elevated in epithelial cells, while TGF-β was not detectable. Conversely, TGF-β levels in the saliva of patients with oropharyngeal candidiasis (OPC) and healthy individuals were low, indicating that oral and vaginal epithelial cells are the primary sources of proinflammatory cytokines in response to C. albicans infection. In a separate study, levels of various cytokines (IL-1β, IL-6, TNF-α, IL-17, IL-22, IL-23, and TGF-β) in the cervicovaginal lavage of a mouse model were measured using the ELISA assay. This study aimed to compare cytokine levels between patients with RVVC, controls, and patients with acute VVC. The results showed that patients with RVVC had higher levels of IL-1β and IL-6 in their cervicovaginal lavage (CVL) compared to controls and also higher IL-6 levels than patients with acute VVC. Tumor necrosis factor-α was not detected in any of the samples. Similar to our study, variations in TGF-β levels were observed among the groups, but these changes did not reach statistical significance (26).
Furthermore, another study highlighted the importance of CMI against VVC in the vaginal tissue of mice. Yano et al. (27) found that suppressed CMI could lead to severe candidiasis in patients. These studies detected high levels of TGF-β in the vaginal tissue of mice and women with VVC, whereas increases in other cytokines were not as pronounced as TGF-β. Thus, TGF-β, as an immunoregulatory cytokine, may clarify the local effectiveness of CMI in the vagina, despite the presence of systemic CMI against candidiasis. Barousse et al. (3) demonstrated a constitutive elevation of TGF-β in adolescents regardless of infection status, while other Th1 and Th2 cytokines remained low.
This suggests that TGF-β, a potent down-regulatory cytokine, might explain the absence of CMI in the vagina despite the presence of Candida-specific systemic CMI. Additionally, animal studies by Taylor et al. (12) aimed to test the hypothesis that CMI at the vaginal mucosa is suppressed in hosts with detectable Candida-specific Th1 immunity at the systemic level. They reported increased gene and protein expression levels of TGF-β1 in mouse vaginal tissue after inoculation with C. albicans, with higher levels in the vaginal tissue compared to other genital areas. They observed a twofold increase in protein level and a tenfold increase in mRNA levels compared to selected Th1 and Th2 cytokines, indicating TGF-β's role in regulating immune response in the vaginal area.
In our study, TGF-β isoform 1 was evaluated as a key mediator in determining the immune response's fate in the vaginal area following Candida infection. Cetyltrimethylammonium bromide (CTAB) mannan-specific and alkaline degradation (PEAT) methods were used to measure C. albicans Mannan specific immunoglobulin G (IgG) and T cell-derived antigen-binding molecules (TABM) in serum samples of women with vulvovaginal candidiasis and controls (21). Immunoglobulin G levels by both methods and TABM levels by CTAB mannan-specific were significantly elevated in patients. TABM had a marked effect on TGF-β2, indicating that cellular immune responses and associated cytokines like IFN-γ and IL-12 are inhibited by TGF-β2.
This suggests that CTAB mannan-specific TABM may increase susceptibility to VVC through a Th2-type immune response. Recent studies have revealed that the three isoforms of TGF-β have different gene encodings and biological capabilities (28, 29). For instance, Lee et al. (29) discovered that the TGF-β3 isoform was more effective at inducing pathogenic TH17 cells compared to TGF-β1. The non-significant result in our study could be due to measuring only TGF-β isoform 1; examining all three isoforms simultaneously in different biological sources and at both gene and protein levels might yield different outcomes. Further studies are needed to explore cytokine and chemokine secretion in mucosal fungal infections thoroughly, providing insights into immunopathogenesis and immunotherapy and offering comprehensive knowledge for treating RVVC or other mucosal candidiasis effectively.
5.1. Conclusions
In summary, several studies have shown that TGF-β plays a significant role in the local immunity of recurrent vulvovaginal candidiasis, with increased expression observed in disease conditions. It is important to note that while the enhancement of TGF-β expression in this study was observed, it did not reach statistical significance.