1. Background
Nosocomial infections are one of the problems in recent decades especially in the wards where immunocompromised patients are hospitalized (1, 2). Bio-aerosols in the hospital air are one of the potential sources of infection (3). Researchers have reported the importance of bio-aerosols as transmitters of nosocomial infections. Microorganisms associated with airborne transmission are bacteria, viruses, and fungi. Airborne micro-fungi in indoor hospital environments are mainly formed by filamentous fungi that belong to the Aspergillus spp., Mucorales (Rhizopus spp.), Fusarium spp., Cladosporium spp., Paecilomyces spp., Scedosporium spp., Penicillium spp., Scopulariopsis spp., Pseudoallescheria boydii, Sporothrix spp. and Acremonium spp. (1, 3, 4). Yeast isolates have also been found that belong to the genus of Candida, Trichosporon, Rhodotorula, Saccharomyces, and Cryptococcus (1, 5-7).
There is little information about how they remain suspended in the air (1). The most common fungal infections with life-threatening complications are aspergillosis and mucormycosis (4, 8). The healthcare features such as overcrowding, unsuitable design, and poor ventilation can impact the growth and proliferation of fungi in building materials, food, pipe leak, bedding, topical anaesthetic, dust, paint, etc., which is harmful to human and animal health (9). Therefore, according to the materials available and other conditions (temperature and humidity), fungal diversity and their concentrations can vary in different departments or hospitals (3, 4, 10).
2. Objectives
This study aimed to assess the fungal diversity and the number of fungal agents in seven wards of educational hospitals of Ahvaz city, Iran during April to July 2016.
3. Methods
3.1. Air Sampling
This study was carried out in seven wards of educational hospitals [surgery, infectious disease, neonatal intensive care unit (NICU), nephrology, intensive care unit (ICU), burn and operating room] in the city of Ahvaz, Iran (Golestan, Abuzar, Imam Khomeini, Taleghani, and Razi hospitals). The air sampling was done during April to July 2016. In each ward, 25 samples were collected and a total of 175 plates were analysed. Air sampling was performed for three minutes using sample pump (Quick Take 30, UK). In daily run, the pump was set for a flux of 28.3 L/min by calibrator (Defender15, UK) (11). Sampling time was 8 A.M. to 14 P.M. In each ward, the Quick Take 30 was located in different areas and it was placed approximately 1.5 meter above the floor and one meter away from an exterior wall to collect the samples (11). Sabouraud dextrose agar (SDA) medium (Merck, Germany) was used for sampling. After sampling, the Petri dishes were closed and sent to the medical mycology laboratory at the Ahvaz Jundishapur University of Medical Sciences.
3.2. Fungal Examinations
Samples were kept at 25°C for 7 to 10 days. After 72 to 96 hours, the number of fungal colonies on SDA was presented in colony-forming unit per cubic meter (CFU/m3). Fungi were grouped to the genus and/or species level by the appearance and microscopic features (11, 12). Identification was conducted based on macroscopic and microscopic features of fungi. After 7 to 10 days when the growth of colonies was completed, direct microscopic examination was performed with Lacto phenol Aniline Blue stain and KOH 10%. Yeasts were identified using morphological characters and cultured on CHROMagar Candida (CHROMagar Candida, France), cornmeal agar (Difco, USA) with tween 80 (Merck, Germany) for enhancement of chlamydospore and maximum growth temperatures on SDA. Slide cultures were prepared for filamentous fungi and finally they were diagnosed with referring to mycological atlases for example, “introduction to food and airborne fungi” compiled by Robert A. Samson et al. (13).
3.3. Statistical Analysis
Data were analysed using SPSS statistical software (version 22) by Mann - Whitney test with the significance level of P < 0.05.
4. Results
The average temperature and relative humidity of indoor air were 26°C (23°C - 28°C) and 38% (32% - 56%), respectively. A total of 2906 fungal colonies (2114 filamentous fungi and 792 yeasts) were isolated from all wards. There were no significant correlations between CFU of fungi with temperature and relative humidity in all of the wards. The fungal isolates belonged to 21 genera (Table 1). Filamentous fungi were found to be 1143 dematiaceous fungi, 930 hyaline fungi, and 38 sterile hyphae. Also, three actinomycetal species were detected. Results showed that the highest concentration of fungi was related to the genus of Cladosporium (35. 3%), followed by yeasts (27.3%) (Rhodotorula, Trichosporon, Schizosaccharomyces, Saccharomyces), Aspergillus (15.1%), Penicillium (12.1%), and others (10.2%).
| Fungus | Number and Percentage of Isolated Fungi | |||||||
|---|---|---|---|---|---|---|---|---|
| Surgery | Infectious | ICU | Nephrology | Burn | Operating | NICU | Total | |
| Cladosporium spp. | 228 (24.1) | 174 (32.9) | 236 (54.4) | 110 (39.3) | 65 (24.6) | 132 (57.1) | 80 (36.4) | 1025 (35.3) |
| Yeast spp. | 466 (49.2) | 174 (32.9) | 21 (4.8) | 22 (7.9) | 91 (34.5) | 4 (1.7) | 14 (6.4) | 792 (27.3) |
| Aspergillus spp. | 146 (15.4) | 43 (8.1) | 54 (12.4) | 58 (20.7) | 36 (13.6) | 45 (19.5) | 57 (25.9) | 439 (15.1) |
| Penicillium spp. | 63 (6.6) | 62 (11.7) | 72 (16.6) | 38 (13.6) | 50 (18.9) | 17 (7.4) | 51(23.2) | 353 (12.1) |
| Alternari spp. | 13 (1.4) | 23 (4.3) | 14 (3.2) | 12 (4.3) | 10 (3.8) | 9 (3.9) | 3 (1.4) | 84 (2.9) |
| Fusarium spp. | 7 (0.7) | 1 (0,2) | 3 (0.7) | 1 (0.4) | - | 2 (0.9) | 14 (0.5) | |
| Rhizopus spp. | 6 (0.6) | 1 (0,2) | 9 (2.1) | 8 (2.9) | 1 (0.4) | 3 (1.3) | 2 (0.9) | 30 (1.0) |
| Chaetomium spp. | - | - | - | 1 (0.4) | - | 1 (0.4) | - | 2 (0.1) |
| Ulocladium spp. | 1 (0.1) | - | - | - | - | 3 (1.3) | - | 4 (0.1) |
| Paecilomyces spp. | 1 (0.1) | 37 (7) | 1 (0.2) | 6 (2.1) | - | 1 (0.4) | 3 (1.4) | 49 (1.7) |
| Acremonium spp. | 1 (0.1) | - | 7 (1.6) | 1 (0.4) | 2 (0.8) | 4 (1.7) | 3 (1.4) | 18 (0.6) |
| Sporotrichum spp. | 4 (0.4) | 2 (0.4) | 2 (0.5) | 9 (3.2) | 2 (0.8) | 4 (1.7) | 2 (0.9) | 25 (0.9) |
| Stemphylium spp. | - | - | - | - | - | 1 (0.4) | 1 (0.5) | 2 (0.1) |
| Curvularia spp. | - | - | - | - | - | - | 1 (0.5) | 1 (0.0) |
| Stachybotrys spp. | - | - | - | 1 (0.4) | 1 (0.4) | 1 (0.4) | - | 3 (0.1) |
| Exophiala spp. | - | - | 1 (0.2) | - | - | - | - | 1 (0.0) |
| Sepedonium spp. | - | - | - | - | 1 (0.4) | - | - | 1 (0.0) |
| Drechsleras spp. | 3 (0.3) | 2 (0.4) | 1 (0.2) | 9 (3.2) | 4 (1.5) | 2 (0.9) | - | 21 (0.7) |
| Actinomycete spp. | 3 (0.3) | - | - | - | - | - | - | 3 (0.1) |
| Sterile hyphae spp. | 6 (0.6) | 10 (1,9) | 13 (3) | 4 (1.4) | 1 (0.4) | 4 (1.7) | - | 38 (1.3) |
| Trichurus spp. | - | - | - | - | - | - | 1 (0.5) | 1 (0.0) |
| Total | 948 (32.6) | 529 (18.2) | 434 (14.9) | 280 (9.6) | 264 (9.1) | 231 (7.9) | 220 (7.6) | 2906 (100) |
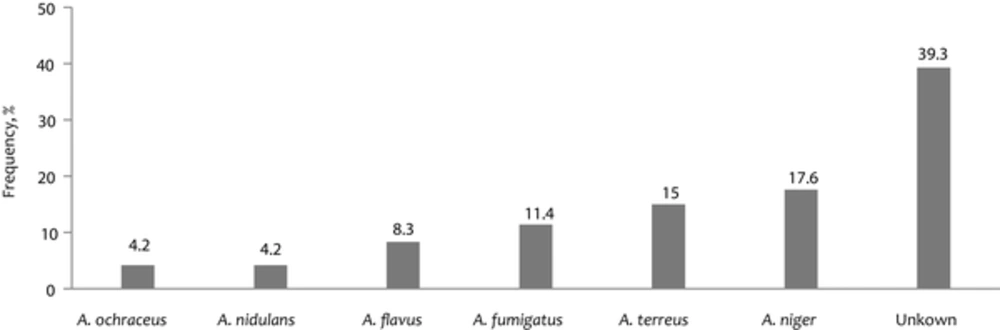
Aspergillus spp. was identified to species level (Figure 1). However, 40% of this genus was not identified at the species level. Total mean of airborne fungal number was 195.59 CFU/m3. Analysis of the fungal bioaerosol concentration in the various wards was shown in different levels of contamination: the lowest and highest values were detected in NICU (103 CFU/m3) and surgery (446 CFU /m3) wards, respectively (Table 2). There was a significant difference in the number of airborne fungi between surgery ward, operating room, and NICU wards (P < 0.05).
| Wards of Sampling | Mean (CFU/m3) (min - max) |
|---|---|
| Surgery | 446 (47 - 5147) |
| Infectious diseases | 249 (47 - 800) |
| ICU | 202 (47 - 565) |
| Nephrology | 131 (58 - 247) |
| Burn | 124 (11 - 318) |
| Operating room | 108 (23 - 259) |
| NICU | 103 (23 - 329) |
5. Discussion
Fungal spores can be readily transported through staff, visitors, windows, patients, and ventilation systems from the outdoors, and grow indoors. They can utilize structural building materials for colonization. Saprophytic fungi are widely distributed in the hospital indoor air, resulting in high morbidity and mortality rates in high-risk patients (14, 15). Invasive fungal infections are the leading cause of morbidity and mortality despite antifungal therapy. In recent decades, nosocomial fungal infections have dramatically increased (16). Previous studies reported that Candida and Aspergillus species were the most predominant causes of nosocomial fungal infections among immunocompromised individuals (16-18), and nosocomial aspergillosis is a common complication among immunocompromised individuals and high-risk patients (19).
The variety of airborne fungi and their concentrations in the indoor air in the selected wards were investigated in this study. The results showed that the pattern of indoor airborne fungi was similar to outdoors, which was proved that the fungal flora in outdoors were the source of the indoor fungi. However, several fungi including Stachybotrys, Sepedonium, Chaetomium, and Ulocladium species were not isolated from outdoors for which, in these cases, a source of indoor fungal origin is suggested. In this study, environments of all wards were contaminated with types of fungi. Several researchers isolated a variety of fungi with different proportions from indoor environments of hospitals (7, 15, 18, 20-25). In this study, among filamentous fungi, Cladosporium spp. were the most dominant fungal isolates followed by Aspergillus spp. and Penicillium spp. In agreement with our results, other investigations performed in Iran revealed the genera of Cladosporium, Penicillium, and Aspergillus as the main fungal isolates (26, 27).
In a study conducted by Hedayati et al. (28) Cladosporium spp., Penicillium spp., and Aspergillus spp. were the most frequent fungi in the indoor air of operating rooms located in Mazandaran province. In another study by Pakshir et al., Cladosporium (32.3%), Aspergillus (23.7%), and Penicillium (9.9%) species were the most common contaminants of indoor air in Fars province (29). Kim et al. (30) recorded Cladosporium spp. (30%), Penicillium spp. (20% - 25%), and Aspergillus spp. (15% - 20%) in a general hospital air. In fact, the above-mentioned fungi produce many light and small spores in outdoor and indoor air. Cladosporium spp. are dematiaceous fungi that grow in high humid conditions. Previous studies demonstrated Cladosporium spp. as the predominant fungi in the indoor air of hospitals. There is little information about nosocomial infections caused by Cladosporium species.
The present study showed that Aspergillus spp. were the second predominant fungal isolates identified. Among Aspergillus species, Aspergillus niger had a higher incidence than other species in all the wards under study, which is consistent with previous studies in Iran (7, 29, 31). No correlations between fungal species and hospital wards, temperature, and relative humidity were observed. The main resources of Aspergillus species in hospitals are dust emissions during hospital construction, unfiltered air ventilation system, carpeting, food, and ornamental plants. The role of Aspergillus species, particularly A. fumigatus, in causing different clinical symptoms ranging from colonization to deep infections in hospitalized and immunocompromised patients reveal the hazards of the presence of these fungi in hospital air (32). In addition, resistance to triazoles, as the mainstay of treatment of A. fumigatus, has been reported (33). Since A. terreus infections have displayed resistance to amphotericin B in vitro and in vivo, and due to the high rate of dissemination of this species in the study; management and more decontamination of hospital air are recommended. Lass-Florl et al. (34) believed the beginning of fungal infection caused by A.terreus depends not on the immunosuppression degree but depends on environmental exposure to Aspergillus spores. In addition, A.terreus is suggested to be associated with hospital plants (35, 36).
There is no invariable standard in world to show tolerable maximum airborne fungi loads. Governmental and private organizations have different values for acceptable fungal bioaerosol concentrations (37, 38). The number of colony forming unit (CFU/m3) varies widely; from < 25 to more than 300. The quantitative interpretation of the results describing the air quality in this study was evaluated based on the American conference of industrial hygienists (ACGIH) standards in 1995. The ACGIH has suggested limits 100 CFU/m3 for fungi in the clean rooms and hospital air (39). Researchers have proposed ranges of 15 CFU/m3 for levels of fungal load and spore count < 0.1 CFU/m3 for A. fumigatus and other saprophytic fungi; in areas where HEPA filter is used with at least 12 air changes per hour (ACH) and positive air pressure (39, 40).
According to these guidelines, all wards that were included in the study were not in hygienic conditions and surgery ward was the most contaminated among the wards special to operating room and NICU wards. These might be because of the number of staff, visitors, and the high density of patients and the presence of a high number of medical students in the wards at the time of our study. Less contamination in the operating room and NICU wards comparing to other wards may be due to the less number of individuals in these wards. Beside these, the environmental factors, mainly, inadequate disinfection, construction, inappropriate and insufficient ventilation system might also contribute to the high fungal loads of the wards.
5.1. Conclusion
As a result, all wards were contaminated with different fungi including filamentous fungi and yeasts. The high fungal concentrations in the indoor air of educational hospitals might be the potential risk factors for the outbreak of nosocomial infections. Thus, it is recommended to control fungal spores’ interventions through installation of good ventilation systems, regular disinfected floors, restoration of the building, systematic checking of the indoor air and ventilation systems, and minimizing commuting.