1. Background
Klebsiella pneumoniae (KP), commonly found in the intestinal and respiratory tracts, is a prevalent gram-negative bacillus that can lead to urinary tract, respiratory tract, and bloodstream infections (1). Bloodstream infections are particularly concerning due to their high incidence and mortality rates, often exacerbated by invasive diagnostic and treatment methods used clinically (2). Klebsiella pneumoniae, a major causative agent of bloodstream infections, can lead to infectious shock if not promptly and effectively treated (3, 4). Although typically treated with potent antibiotics like carbapenems, KP has increasingly become resistant to many drugs due to the extensive use of broad-spectrum antibiotics (5). This resistance, especially to carbapenems carbapenem-resistant Klebsiella pneumoniae (CRKP), restricts treatment options and complicates management (6). Therefore, understanding the clinical features of CRKP-induced bloodstream infections is crucial for improving outcomes and treatment success rates. While research on CRKP-induced bloodstream infections predominantly involves adults (7), there is a significant need to explore these aspects in children.
2. Objectives
This study aimed to analyze the risk factors for mortality in children with CRKP-induced bloodstream infections using a multivariate logistic regression model, and to discuss their clinical characteristics to aid in better control, early diagnosis, and treatment.
3. Methods
3.1. General Data
We collected clinical data from 160 children treated for CRKP-induced bloodstream infections in our hospital between January 2020 and January 2023. The cohort included 76 boys and 84 girls, averaging an age of 9.16 ± 6.13 years. The death group (n = 61) consisted of 29 boys and 32 girls, averaging 9.78 ± 8.45 years old, with a BMI of 16.18 ± 2.54 kg/m2, and a hospital stay of 22.45 ± 4.89 days. The survival group included 47 boys and 52 girls, averaging 8.46 ± 8.33 years old, with a BMI of 16.45 ± 2.87 kg/m2, and a hospital stay of 39.74 ± 6.84 days.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria were: (1) children meeting the diagnostic criteria for bloodstream infections (8), (2) those identified with CRKP as defined by the CDC Guidelines for the Control of Carbapenem-Resistant Enterobacteriaceae (9, 10), and (3) those with at least one positive blood culture result after admission. Exclusion criteria included: (1) Children admitted for less than 24 hours, (2) those with poor compliance, or (3) those with incomplete clinical data.
Determination criteria for death in children with CRKP-induced bloodstream infections and grouping methods. All children with CRKP-induced bloodstream infections in our hospital received 20 mg/kg of meropenem intravenously, diluted in 10 mL of 0.9% sodium chloride injection (Sinopharm Pharmaceutical Co., Ltd., China), administered every 8 hours. Based on outcomes 30 days post-infection, patients were categorized into a death group or a survival group, with death serving as the endpoint event.
3.3. Clinical Data Collection
3.3.1. Baseline Data
We recorded gender, age, BMI, length of hospital stay, whether the child was an only child, place of residence, primary caregiver, and complications (such as sepsis, malignant tumors, renal, cardiovascular, and cerebrovascular diseases) using the hospital's medical record system.
3.3.2. Laboratory Indicators
We collected 5 mL of fasting venous blood from each child, which was then centrifuged to obtain serum. The levels of platelets, hemoglobin, white blood cells, red blood cells, and procalcitonin were measured using an automatic biochemical analyzer (Olympus, Japan).
3.3.3. Scale Evaluation
The severity of the children's conditions was assessed using the acute physiology and chronic health evaluation II (APACHE II) score, which totals 71 points, with higher scores indicating more severe conditions.
3.3.4. Bloodstream Infections
These were categorized into lower respiratory tract infection, urinary tract infection, surgical site infection, and abdominal infection based on the infection site.
3.4. Antimicrobial Susceptibility Testing
Blood cultures from 160 CRKP strains were conducted, followed by strain identification using a microbial identification time-of-flight mass spectrometer (Xiamen microTyper, China). Antimicrobial susceptibility results were obtained using the Phoenix-100 bacterial identifier (BD, USA) and interpreted according to the NCCLS Standards for Antimicrobial Susceptibility Testing (11).
3.5. Statistical Analysis
Data analysis was performed using SPSS version 26.0 (IBM Inc., USA). Measurement data normality was assessed. Normally distributed data were expressed as mean ± standard deviation (
4. Results
4.1. Antimicrobial Susceptibility Testing Results of 160 Carbapenem-Resistant <i>Klebsiella pneumoniae</i> Strains
Antimicrobial susceptibility testing revealed that the strains were highly resistant to aztreonam, ceftriaxone, ampicillin-sulbactam, ampicillin, meropenem, imipenem, cefoperazone-sulbactam, piperacillin-tazobactam, levofloxacin, and ciprofloxacin. However, they showed high sensitivity to ceftazidime-avibactam and polymyxin B (Table 1).
| Antimicrobial Drug | Number of Strains | Sensitive | Intermediate | Resistant |
|---|---|---|---|---|
| Aztreonam | 147 | 8 (5.44) | 0 (0.00) | 139 (94.56) |
| Ceftriaxone | 160 | 0 (0.00) | 0 (0.00) | 160 (100.00) |
| Ampicillin-sulbactam | 160 | 0 (0.00) | 0 (0.00) | 160 (100.00) |
| Ampicillin | 160 | 0 (0.00) | 0 (0.00) | 160 (100.00) |
| Meropenem | 119 | 7 (5.88) | 0 (0.00) | 112 (94.12) |
| Imipenem | 160 | 10 (6.25) | 0 (0.00) | 150 (93.75) |
| Cefoperazone-sulbactam | 138 | 6 (4.35) | 2 (1.45) | 130 (94.20) |
| Piperacillin-tazobactam | 160 | 10 (6.25) | 7 (4.38) | 143 (89.38) |
| Amikacin | 160 | 59 (36.88) | 0 (0.00) | 101 (63.12) |
| Gentamicin | 160 | 29 (18.13) | 5 (3.13) | 126 (78.75) |
| Tobramycin | 160 | 28 (17.50) | 14 (8.75) | 118 (73.75) |
| Ceftazidime-avibactam | 35 | 35 (100.00) | 0 (0.00) | 0 (0.00) |
| Polymyxin B | 53 | 50 (94.34) | 1 (1.87) | 2 (3.77) |
| Fosfomycin | 98 | 9 (9.18) | 12 (12.24) | 77 (78.57) |
| Minocycline | 120 | 102 (85.00) | 10 (8.33) | 8 (6.67) |
| Tigecycline | 122 | 118 (96.72) | 2 (1.64) | 2 (1.64) |
| Compound sulfamethoxazole | 160 | 86 (53.75) | 0 (0.00) | 74 (46.25) |
| Levofloxacin | 160 | 12 (7.50) | 10 (6.25) | 138 (86.25) |
| Ciprofloxacin | 160 | 8 (5.00) | 9 (5.63) | 143 (89.37) |
Antimicrobial Susceptibility Testing Results of 160 Carbapenem-Resistant Klebsiella pneumoniae Strains a
4.2. Clinical Symptoms of Children with Carbapenem-Resistant <i>Klebsiella pneumoniae</i>-Induced Bloodstream Infections
The predominant clinical symptoms in children with CRKP-induced bloodstream infections included fever, chills, fatigue, dizziness, dyspnea, mental status changes, and cough. Fever and dizziness were the most common symptoms (Table 2).
| Clinical Symptoms | Values |
|---|---|
| Fever | 123 (76.88) |
| Chills | 98 (61.25) |
| Fatigue | 81 (50.63) |
| Dizziness | 135 (84.38) |
| Dyspnea | 64 (40.00) |
| Change in mental status | 79 (49.38) |
| Cough | 58 (36.25) |
Clinical Symptoms of Children with Carbapenem-Resistant Klebsiella pneumoniae-Induced Bloodstream Infections a
4.3. Outcomes of Children with Carbapenem-Resistant <i>Klebsiella pneumoniae</i>-Induced Bloodstream Infections
Out of 160 children with CRKP-induced bloodstream infections treated at our hospital, 99 survived, representing 61.87% of the cases. The remaining 61 children died, accounting for 38.13%.
4.4. Clinical Data of Survival and Death Groups
The analysis of clinical data between the survival and death groups showed no significant differences in gender, age, BMI, only child status, usual residence, primary caregiver, cardiovascular diseases, cerebrovascular diseases, platelets, hemoglobin, white blood cells, red blood cells, lower respiratory tract infection, urinary tract infection, and abdominal infection (P > 0.05). However, the death group had a shorter hospital stay and higher incidence rates of sepsis, malignant tumors, renal diseases, infectious shock, surgical site infection, APACHE II scores, and procalcitonin levels compared to the survival group (P < 0.05) (Table 3).
| Parameter | Death Group (n = 61) | Survival Group (n = 99) | Statistical Value | P-Value |
|---|---|---|---|---|
| Gender | 0.003 | 0.959 | ||
| Male | 29 (47.54) | 47 (47.47) | ||
| Female | 32 (52.46) | 52 (52.53) | ||
| Age ( | 9.78 ± 8.45 | 8.46 ± 8.33 | 0.968 | 0.334 |
| BMI ( | 16.18 ± 2.54 | 16.45 ± 2.87 | 0.603 | 0.547 |
| Length of hospital stay | 22.45 ± 4.89 | 39.74 ± 6.84 | 16.554 | 0.000 |
| Only child | 0.850 | 0.357 | ||
| Yes | 22 (36.07) | 43 (43.43) | ||
| No | 39 (63.93) | 56 (56.57) | ||
| Place of usual residence | 0.572 | 0.450 | ||
| Rural | 21 (34.43) | 40 (40.40) | ||
| Urban | 40 (65.57) | 59 (59.60) | ||
| Primary caregiver | 1.961 | 0.161 | ||
| Relatives | 47 (77.05) | 66 (66.67) | ||
| Others | 14 (22.95) | 33 (33.33) | ||
| Sepsis | 5.795 | 0.016 | ||
| Yes | 13 (21.31) | 8 (8.08) | ||
| No | 48 (78.69) | 91 (91.92) | ||
| Malignant tumors | 11.116 | 0.001 | ||
| Yes | 43 (70.49) | 43 (43.43) | ||
| No | 18 (29.51) | 56 (56.57) | ||
| Renal diseases | 16.860 | 0.000 | ||
| Yes | 40 (65.57) | 32 (32.32) | ||
| No | 21 (34.43) | 67 (67.68) | ||
| Cardiovascular diseases | 0.014 | 0.908 | ||
| Yes | 32 (52.46) | 51 (51.52) | ||
| No | 29 (47.54) | 48 (48.48) | ||
| Cerebrovascular diseases | 1.374 | 0.241 | ||
| Yes | 15 (24.59) | 33 (33.33) | ||
| No | 46 (75.41) | 66 (66.67) | ||
| Platelets ( | 210.47 ± 31.86 | 220.49 ± 42.58 | 1.524 | 0.130 |
| Hemoglobin ( | 134.82 ± 12.71 | 139.12 ± 13.89 | 1.892 | 0.060 |
| White blood cells ( | 7.16 ± 3.14 | 7.30 ± 3.66 | 0.239 | 0.812 |
| Red blood cells ( | 4.61 ± 1.50 | 4.48 ± 1.46 | 0.518 | 0.605 |
| Procalcitonin ( | 29.87 ± 7.56 | 19.81 ± 9.03 | 7.000 | 0.000 |
| Acute physiology and chronic health evaluation II score | 23.19 ± 5.61 | 16.75 ± 5.13 | 7.180 | 0.000 |
| Infectious shock | 10.678 | 0.001 | ||
| Yes | 39 (63.93) | 37 (37.37) | ||
| No | 22 (36.07) | 62 (62.63) | ||
| Lower respiratory tract infection | 0.029 | 0.864 | ||
| Yes | 23 (37.70) | 36 (36.36) | ||
| No | 38 (62.30) | 63 (63.64) | ||
| Urinary tract infection | 0.163 | 0.686 | ||
| Yes | 26 (42.62) | 39 (39.39) | ||
| No | 35 (57.38) | 60 (60.61) | ||
| Surgical site infection | 6.349 | 0.012 | ||
| Yes | 39 (63.93) | 43 (43.43) | ||
| No | 22 (36.07) | 56 (56.57) | ||
| Abdominal infection | 0.014 | 0.905 | ||
| Yes | 21 (34.43) | 35 (35.35) | ||
| No | 40 (65.57) | 64 (64.65) |
Clinical Data of Survival and Death Groups a
4.5. Results of Multivariate Logistic Regression Analysis of Risk Factors for Death in Children with Carbapenem-Resistant <i>Klebsiella pneumoniae</i>-Induced Bloodstream Infections
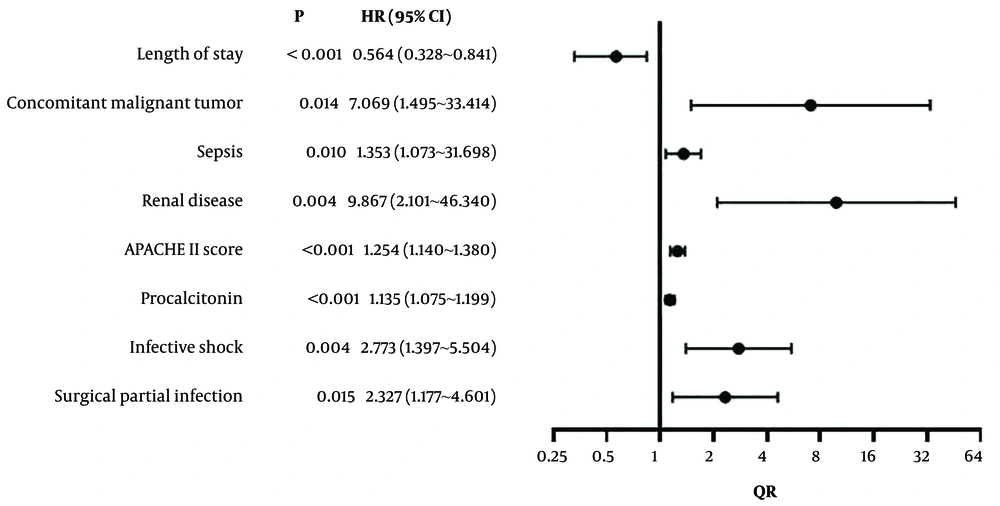
Logistic regression analysis used the mortality of children with CRKP-induced bloodstream infections as the dependent variable (death group = 1, survival group = 0), with significant clinical indicators (hospital stay length, malignant tumors, sepsis, renal diseases, APACHE II score, procalcitonin, infectious shock, surgical site infection) as independent variables. These variables are detailed in Table 4. The analysis identified that hospital stay length, malignant tumors, sepsis, renal diseases, APACHE II score, procalcitonin, and infectious shock were independent risk factors for mortality (P < 0.05) (Table 5 and Figure 1).
| Variables | Meaning | Assignment |
|---|---|---|
| X1 | Length of hospital stay | Continuous variable |
| X2 | Malignant tumors | Yes = 1, No = 0 |
| X3 | Sepsis | Yes = 1, No = 0 |
| X4 | Renal diseases | Yes = 1, No = 0 |
| X5 | Acute physiology and chronic health evaluation II score | Continuous variable |
| X6 | Procalcitonin | Continuous variable |
| X7 | Infectious shock | Yes = 1, No = 0 |
| X8 | Surgical site infection | Yes = 1, No = 0 |
| Y | Death of children with carbapenem-resistant Klebsiella pneumoniae -induced bloodstream infections | Death = 1, Survival = 0 |
Assignment of Variables
| Item | B | Standard Error | Wald | P-Value | OR | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Length of hospital stay | -0.447 | 0.083 | 28.807 | 0.000 | 0.564 | 0.328 - 0.841 |
| Malignant tumors | 1.956 | 0.792 | 6.090 | 0.014 | 7.069 | 1.495 - 33.414 |
| Sepsis | 1.041 | 0.801 | 1.689 | 0.010 | 1.353 | 1.073 - 1.698 |
| Renal diseases | 2.289 | 0.789 | 8.413 | 0.004 | 9.867 | 2.101 - 46.340 |
| Acute physiology and chronic health evaluationn II score | 0.226 | 0.049 | 21.558 | 0.000 | 1.254 | 1.140 - 1.380 |
| Procalcitonin | 0.127 | 0.028 | 20.898 | 0.000 | 1.135 | 1.075 - 1.199 |
| Infectious shock | 1.020 | 0.350 | 8.502 | 0.004 | 2.773 | 1.397 - 5.504 |
| Surgical site infection | 0.845 | 0.348 | 5.892 | 0.015 | 2.327 | 1.177 - 4.601 |
Results of Multivariate Logistic Regression Analysis of Risk Factors for Death of Children with Carbapenem-Resistant Klebsiella pneumoniae-Induced Bloodstream Infections
4.6. Receiver Operating Characteristic Curves for Factors Affecting Mortality in Children with Carbapenem-Resistant <i>Klebsiella pneumoniae</i>-Induced Bloodstream Infections
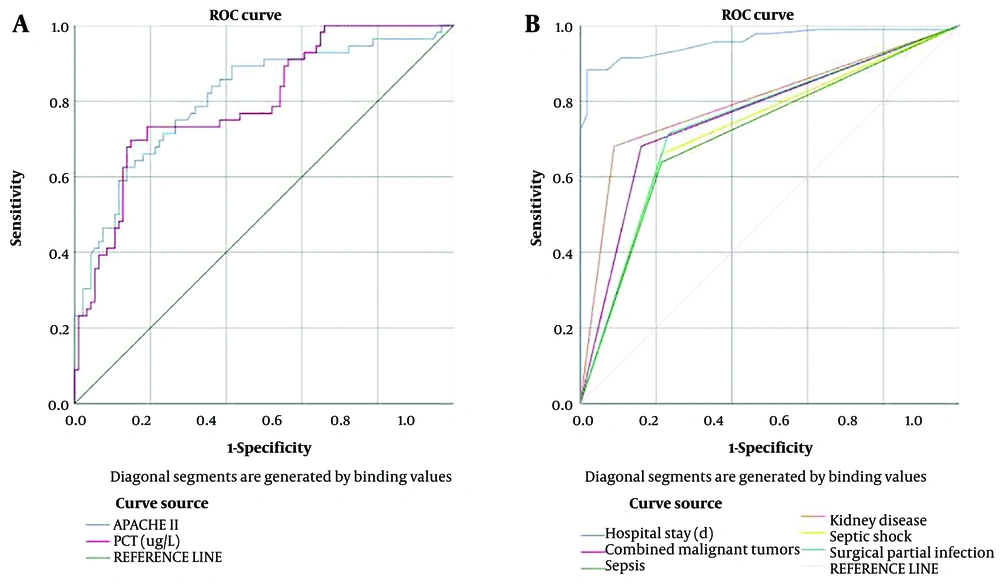
Receiver operating characteristic curves were used to evaluate the predictive value of various clinical indicators for mortality in children with CRKP-induced bloodstream infections, with the death of children as the dependent variable (death group = 1, survival group = 0). The significant clinical indicators included the length of hospital stay, malignant tumors, sepsis, renal diseases, APACHE II score, procalcitonin, infectious shock, and surgical site infection. The areas under the ROC curves (AUC) for these indicators were all greater than 0.700, demonstrating their strong predictive value for mortality (Table 6 and Figure 2).
| Parameter | AUC | Cut-off Value | 95% CI | P-Value | Specificity | Sensitivity | Youden Index |
|---|---|---|---|---|---|---|---|
| Length of hospital stay | 0.957 | 29.500 | 0.927 - 0.988 | 0.000 | 0.839 | 0.915 | 0.754 |
| Malignant tumors | 0.760 | 1.500 | 0.681 - 0.840 | 0.000 | 0.839 | 0.681 | 0.520 |
| Sepsis | 0.712 | 1.500 | 0.627 - 0.797 | 0.000 | 0.786 | 0.638 | 0.424 |
| Renal diseases | 0.796 | 1.500 | 0.723 - 0.869 | 0.000 | 0.911 | 0.681 | 0.592 |
| Acute physiology and chronic health evaluationn II score | 0.811 | 19.375 | 0.737 - 0.884 | 0.000 | 0.660 | 0.786 | 0.446 |
| Procalcitonin | 0.796 | 22.345 | 0.722 - 0.869 | 0.000 | 0.479 | 0.768 | 0.247 |
| Infectious shock | 0.723 | 1.500 | 0.638 - 0.807 | 0.000 | 0.786 | 0.660 | 0.426 |
| Surgical site infection | 0.740 | 1.500 | 0.657 - 0.824 | 0.000 | 0.768 | 0.713 | 0.481 |
Results of Receiver Operating Characteristic Curve Analysis of Factors Influencing Death of Children with Carbapenem-Resistant Klebsiella pneumoniae-Induced Bloodstream Infections
5. Discussion
Carbapenem-resistant K. pneumoniae is increasingly prevalent, with resistance rates to many effective antibiotics exceeding 40%, complicating treatment and increasing mortality rates from CRKP-induced bloodstream infections (12). In this study, 61 of the 160 children with CRKP-induced bloodstream infections admitted to our hospital died, representing 38.13% of cases, consistent with the noted high mortality rate. Factors such as the length of hospital stay, malignant tumors, sepsis, renal diseases, APACHE II score, procalcitonin levels, infectious shock, and surgical site infections showed significant differences, indicating their relevance to mortality in these infections. Logistic regression analysis revealed that a longer hospital stay was a protective factor against death, while the presence of malignant tumors, sepsis, renal diseases, infectious shock, surgical site infections, a high APACHE II score, and high procalcitonin levels were independent risk factors for mortality.
Potential reasons include the following: Prolonged hospital stays allow for close monitoring of CRKP-induced bloodstream infections, timely adjustments in the face of antibiotic resistance, and early interventions that may reduce the risk of additional infections (13). Children with malignant tumors often undergo aggressive treatments like chemotherapy, radiotherapy, hormone therapy, and surgical resections that may compromise mitochondrial function and subsequently impair cell metabolism and immune responses, exacerbating their condition in the event of infections (14). Furthermore, tumors may locally infiltrate, destroying the natural defense barriers of tissues and increasing both the likelihood of infections and the risk of mortality (15).
In recent years, there has been a significant increase in the resistance of septic patients to antibacterial drugs, including carbapenems, which complicates the condition of children with CRKP (16). In cases of sepsis, various pathogenic bacteria enter the bloodstream, releasing toxins and metabolites that impair hemoglobin's oxygen-transport capacity, thereby damaging organ functions and exacerbating CRKP infections (17). Additionally, children with renal diseases experience more severe conditions during bloodstream infections due to compromised detoxification functions (18). In this study, the APACHE II scores were higher in the death group than in the survival group, aligning with findings from previous literature (19).
Furthermore, procalcitonin levels are directly proportional to the severity of bacterial infections (20). This study found that procalcitonin levels were significantly higher in the death group than in the survival group, consistent with previous research. Infectious shock, a critical systemic condition, is a major cause of mortality in patients with CRKP-induced bloodstream infections. It rapidly progresses, severely impairing vital organs and causing immune function abnormalities (21). Children with surgical site infections may experience systemic inflammatory responses triggered by bacteria or inflammatory factors entering the bloodstream, leading to complications such as sepsis and septicemia, which increase treatment difficulty and mortality (22).
The results of ROC curve analysis in this study showed that the AUC values for length of hospital stay, malignant tumors, sepsis, renal diseases, APACHE II score, procalcitonin, infectious shock, and surgical site infection were all greater than 0.700, indicating these indicators' strong predictive value for mortality in children with CRKP-induced bloodstream infections.
5.1. Conclusions
The mortality rate is high among children with CRKP-induced bloodstream infections. Factors such as a short hospital stay, the presence of malignant tumors, sepsis, renal diseases, infectious shock, high APACHE II scores, and high procalcitonin levels are independent risk factors for death. These indicators can be utilized to improve prognosis as early as possible in clinical treatments.