1. Background
COVID-19 was first identified in late 2019. As of August 21, 2023, the World Health Organization (WHO) has reported 761,071,826 confirmed global cases of COVID-19 and 6,879,677 deaths (1). Systematic analyses have estimated the actual mortality of the pandemic to be around 12-22 million cases (2). Therefore, further investigations are necessary to elucidate the relationships between excess mortality due to SARS-CoV-2 and other indirect factors of various origins (3). Numerous studies have demonstrated that protective antibodies generated through vaccination or natural infection play a crucial role in restraining the spread of the infection. Vaccination against SARS-CoV-2 has been a cornerstone of control strategies. Once initiated, the adaptive immune system orchestrates the elimination of infected cells, triggers B lymphocytes to produce specific antibodies, and establishes immunological memory. The significant role of nutrition in defending against respiratory viral infections, such as influenza and COVID-19, has been well-established (4).
Several nutrients and nutraceuticals, including vitamins and trace elements, serve essential and complementary roles in enhancing innate and adaptive immune responses. Deficiency and insufficiency of these components can have detrimental effects on the proper functioning of the immune system (5). Inadequate data exist regarding micronutrient insufficiency levels, which may contribute to susceptibility to various infections. Moreover, recommended dietary allowances (RDAs) for these components may vary among countries, particularly in the developing world, leading to varying disease burdens accordingly.
Vitamins play vital roles in immune function, from physical and biochemical barriers to innate and adaptive immunity (6). Severe vitamin C deficiency was the first micronutrient recognized to contribute to scurvy, a condition characterized by impaired immunity (7). Vitamin C's antioxidant role is of significant interest, as research has demonstrated its ability to neutralize excessive free radical molecules that can damage cells. Additionally, vitamin C plays a crucial role in the body's immune system by stimulating white blood cell activity. Both vitamins C and D are well-established as micronutrients that support the immune system (6). There have been reports suggesting that these vitamins may help prevent respiratory infections and reduce the severity of COVID-19 symptoms, especially in individuals without comorbidities (8). However, the effectiveness of vitamin C supplementation as adjunctive therapy in reducing complications in critically ill COVID-19 patients has been a subject of controversy (9, 10).
Vitamin D can modulate both innate and adaptive immune responses. Deficiency in vitamin D is associated with an increased risk of autoimmunity and susceptibility to infections. Some studies have recognized vitamin E as a significant component of the body's antioxidant defense system, protecting cell membrane integrity against free radicals through various antioxidant pathways. Vitamin E contributes to maintaining a robust immune system capable of defending against viruses and bacteria. Additionally, it aids in red blood cell formation and widens blood vessels to prevent clotting, facilitating intercellular communication (11-13). While several studies suggest potential adverse effects of vitamin E in non-infectious diseases, such as cardiovascular diseases and cancer, it has been reported to enhance cellular defense against infectious diseases, including influenza and COVID-19 (14). Vitamins C, D, and E collectively stimulate Th2 responses, thereby promoting humoral immunity.
2. Objectives
Given the potential roles of vitamins C, D, and E in the management of COVID-19, this study aimed to investigate the serum levels of these vitamins in individuals who had previously been vaccinated against COVID-19 and experienced disease relapse. Two groups of patients, one hospitalized in regular wards and the other in the intensive care unit (ICU) of the hospital, were included in the study to assess the levels of vitamins C, D, and E. Their results were then compared with those of non-COVID-19 patients.
3. Methods
3.1. Study Design and Population
In this case-control study, a total of 300 subjects (148 males and 142 females) with a mean age of 47.21 years, who had received 2 doses of the vaccine, were admitted to Ayatollah Kashani Hospital in Tehran, Iran. The cases consisted of 100 patients hospitalized in the ICU (ICU group) with severe COVID-19, 100 patients hospitalized in the regular wards with non-severe disease (ward group), and a control group of 100 individuals. Pharyngeal swab specimens were collected from all participants for the SARS-CoV-2 PCR test (SANSURE Novel Coronavirus Nucleic Acid Diagnostic Kit). Those who tested positive for COVID-19 had 10 cc of blood samples taken in tubes without anticoagulants for further testing.
3.2. COVID-19 IgM and IgG Detection
IgM and IgG antibodies were quantified using antibody detection ELISA kits against SARS-CoV-2 from Padtan Gostar Issar Company in Tehran, Iran. The assays were performed following the instruction manuals of the kits, with a sensitivity of 81.82, a specificity of 94.83, and a standard range of positive > 1.1.
3.3. Quantification of 25-Hydroxy Vitamin D3
Vitamin D levels were quantified using a 25-Hydroxyvitamin D3 competitive ELISA kit from Pishtaz Teb Company in Tehran, Iran. All assay steps followed the manufacturer's instructions, with a sensitivity of 96.56, a specificity of 99.8, and a standard range of 30 - 70 ng/mL.
3.4. Quantification of Vitamins C and E
The Abbexa Vitamin ELISA kit (Cambridge, UK) utilizes competitive enzyme-linked immunoassay technology. In this kit, vitamin C (with a sensitivity of 97.56 and a specificity of 98.4 and normal range of 1.2 - 2.0 ng/mL) (ascorbic acid) and vitamin E (with a sensitivity of 97.56 and a specificity of 98.4 and normal range of 5.5 - 17 ng/mL) (alpha-tocopherol) were previously coated with antibodies against each in a 96-well plate. Standards, test samples, and biotin conjugation reagents were added to the wells and incubated following the manufacturer's instructions. A competitive inhibition reaction occurred between biotin-labeled and unlabeled vitamin molecules and was performed on the coated antibodies. The test was carried out according to the manufacturer's instructions.
3.5. Statistical Analysis
All statistical analyses were conducted using SPSS statistics software version 26.0. Descriptive analysis was reported as mean ± SD, median, and interquartile range (IQR). The normality of variables was assessed using the Shapiro-Wilk test. The Student t-test was employed to compare variables with a normal distribution between two groups, while the Mann-Whitney U test was used to compare variables without a normal distribution. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were utilized to determine the optimal cut-off for predicting the best biomarker among ICU and ward patients.
4. Results
4.1. Levels of Vitamins C, D, and E Differ Between Hospitalized and Healthy Individuals
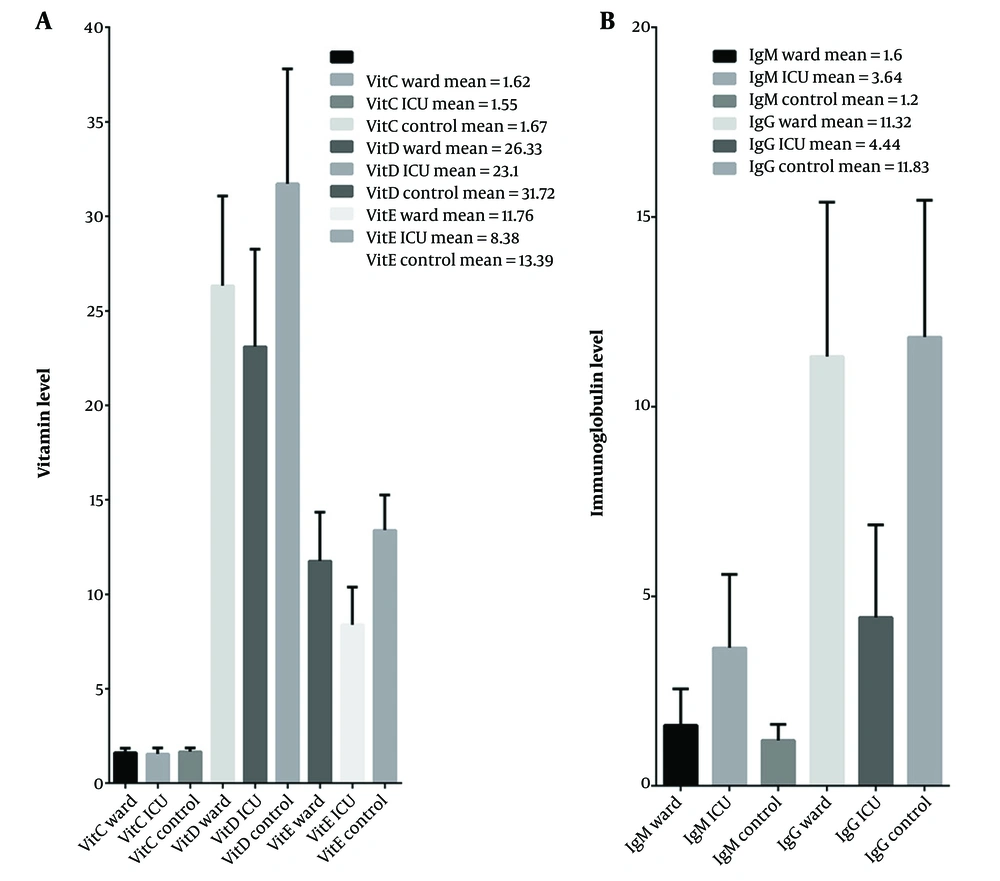
The three groups exhibited variations in serum vitamin levels corresponding to the severity of the disease. The levels of vitamin C were significantly lower in ICU patients compared to the control group (P-value < 0.05). However, no significant difference was observed between the other groups, neither between the ward and control groups nor between the ward and ICU groups. The average serum levels of vitamins D and E were significantly lower among ICU patients than among those hospitalized in the ward. Furthermore, the serum levels of vitamins D and E were significantly higher in the control group compared to the patient groups (Table 1 and Figure 1A).
| Vitamin and Immunoglobulin Levels | ICU vs. Ward | ICU vs. Control | Ward vs. Control |
|---|---|---|---|
| Vit C, ng/mL | |||
| P-value | 0.06 | < 0.05 | 0.08 |
| Value | 1.58 ± 0.069 | 1.61 ± 0.084 | 1.79 ± 0.17 |
| Vit D, ng/mL | |||
| P-value | < 0.001 | < 0.001 | P < 0.001 |
| Value | 24.71 ± 2.28 | 27.41 ± 6.09 | 29.02 ± 3.8 |
| Vit E, ng/mL | |||
| P-value | < 0.001 | < 0.001 | < 0.001 |
| Value | 10.07 ± 2.39 | 10.88 ± 3.54 | 12.57 ± 1.15 |
| IgM, g/L | |||
| P-value | < 0.001 | < 0.001 | < 0.01 |
| Value | 2.62 ± 1.44 | 2.42 ± 1.72 | 1.4 ± 0.28 |
| IgG, g/L | |||
| P-value | < 0.001 | < 0.001 | 0.13 |
| Value | 7.88 ± 4.86 | 8.13 ± 5.22 | 11.57 ± 0.36 |
a Values are expressed as mean ± SD.
Comparison of immunoglobulin (positive > 1.1 g/L) and vitamin (ng/mL) levels among patients and healthy individuals. The mean of each bar is presented in the figures. Mean vitamin levels decreased inversely with the disease severity; B, The mean IgM levels were highest in the ICU group and lowest in the control group; conversely, the IgG levels were highest in the control group and lowest in ICU patients.
Patients with vitamin D and C deficiencies had higher numbers among ICU patients than among ward and control individuals, respectively (P-value < 0.001). The frequency of individuals with a deficiency in all three vitamins was significantly higher among ICU patients than in the hospital ward (P-value < 0.05). The percentage of individuals with triple vitamin deficiency was lowest among the control group (Table 2). When calculating the ratios between different vitamins, statistically significant differences were observed among the three groups under study. In the case of the D-to-C vitamin ratio, the ward group showed a significantly higher ratio than the ICU group (P-value = 0.019). Similarly, the E-to-C and D-to-E vitamin ratios significantly differed between patients (P-value = 0.000) (Table 3).
| Variables | Ward | ICU | Control | Total, No. (%) |
|---|---|---|---|---|
| Vitamins D, E, and C | ||||
| Non-Deficient | 99 | 94 | 100 | 293 (97.66) |
| Deficient | 1 | 6 | 0 | 7 (2.33) |
| Vitamins D and C | ||||
| Non-Deficient | 98 | 87 | 99 | 284 (94.66) |
| Deficient | 2 | 13 | 1 | 16 (5.33) |
| Vitamins E and C | ||||
| Non-Deficient | 99 | 96 | 100 | 295 (98.33) |
| Deficient | 1 | 4 | 0 | 5 (1.66) |
| Variables | VitD/VitC | VitE/VitC | VitD/VitE | IgG/IgM |
|---|---|---|---|---|
| All cases | Ctrl > Ward > ICU | Ctrl > Ward > ICU | ICU > Ctrl > Ward | Ctrl > Ward > ICU |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 |
| Severe and non-severe patients | Ward > ICU | Ward > ICU | ICU > Ward | Ward > ICU |
| P-value | 0.019 | 0.000 | 0.000 | 0.000 |
a P-values < 0.05 indicate significant differences.
b P-values were obtained from the student t-test and the Mann-Whitney U test.
4.2. Serum IgM and IgG Levels Significantly Differ Based on Disease Severity
The results indicated that the IgM levels were directly proportional to the disease severity. IgM levels were significantly higher in the ICU group compared to the ward and control groups, as shown in Figure 1B. Additionally, the number of IgM-positive individuals was highest among the ICU patients, as indicated in Table 4. On average, the number of individuals with positive serum IgM was significantly higher among patients in the hospital ward compared to the control group.
| Variables | Ward | ICU | Control | Total, No. (%) |
|---|---|---|---|---|
| IgG | ||||
| Negative | 3 | 20 | 1 | 24 (8) |
| Positive | 97 | 80 | 99 | 276 (92) |
| IgM | ||||
| Negative | 76 | 14 | 99 | 189 (63) |
| Positive | 24 | 86 | 1 | 111 (37) |
Conversely, the average serum IgG levels were inversely proportional to the disease severity. IgG levels were significantly lower among ICU patients compared to patients in the hospital ward and the control group (P-value < 0.001). However, there was no significant difference between the ward and control groups in terms of IgG levels. IgG-positive individuals were significantly more prevalent in the control group compared to the ward group, and their prevalence was the lowest among ICU patients. Similarly, the IgG-to-IgM ratio showed a significant decrease in ICU patients compared to the ward group, and the ward group showed a significant decrease compared to the control individuals.
4.3. Relationship Between Vitamin and Serum IgG and IgM Levels
The results regarding IgM levels in patient groups indicated that patients with sufficient vitamin E had significantly lower IgM levels than those with insufficient vitamin E (P-value < 0.01). A similar relationship was observed when assessing the relationship between IgM and vitamin E among all participants in the study (P-value < 0.001). However, no significant difference in serum IgM was observed among the groups under study in the case of vitamins C and D.
Patients with normal vitamin C levels had significantly lower IgG levels (P-value = 0.007). Among all studied cases, those with vitamin D insufficiency exhibited lower IgG levels (P-value < 0.001). Regarding vitamin E, patients with different vitamin levels showed no significant differences in serum IgG levels. However, the overall evaluation of the relationship between vitamins and serum immunoglobulin levels among all groups revealed that IgG and IgM levels significantly differed in relation to vitamin statuses (Table 4).
4.4. Association of Vaccine Type with Serum IgG and IgM Levels
All participants had received two doses of either AstraZeneca or Sinopharm vaccines before participating in the current study. The average levels of anti-SARS-CoV-2 IgM and IgG among different groups were examined. However, no significant association between vaccine type and immunoglobulin levels was observed. Similarly, no significant difference was observed between the two groups of patients in the ward and ICU regarding the association between vaccine type and immunoglobulin levels. Interestingly, when evaluating patients based on IgM and IgG status (positive or negative for IgM), the percentage of IgM-positive individuals was significantly higher among individuals immunized with Sinopharm than patients who had received the AstraZeneca vaccine (AstraZeneca: 31 persons, Sinopharm: 169 persons). However, no significant association with IgG status was detected.
4.5. Investigation of Disease Biomarkers
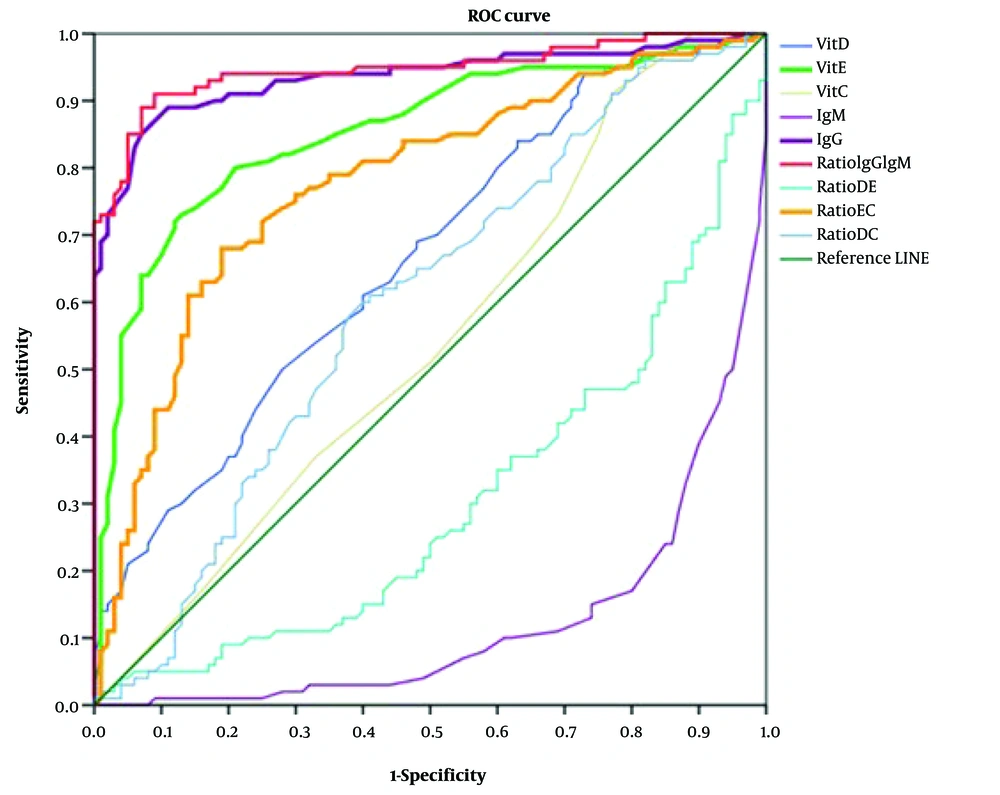
Receiver operating characteristic curves were used to assess the sensitivity and specificity of laboratory tests for disease diagnosis. The AUC and P-values were calculated to identify the best biomarker for the disease. IgM (AUC = 0.783, P-value = 0.000) and the Vit D/Vit E ratio (AUC = 0.573, P-value = 0.000) were identified as the best biomarkers for distinguishing between patients and healthy individuals. IgM (AUC = 0.953, P-value = 0.000) and IgG (AUC = 0.684, P-value 0.000) were also found to be suitable biomarkers for comparing ICU patients with healthy subjects. Among ward and healthy cases, IgM (AUC = 0.613, P-value = 0.006) served as a satisfactory biomarker. In the comparison between ward and ICU patients, the IgG/IgM ratio (AUC = 0.947, P-value = 0.000) and IgG levels (AUC = 0.935, P-value = 0.000) were identified as the best biomarkers, followed by Vit E levels (AUC = 0.853, P-value = 0.000), the Vit E/Vit C ratio (AUC = 0.777, P-value = 0.000), and Vit D levels (AUC = 0.664, P-value = 0.000) (Figure 2).
Receiver operating characteristic (ROC) analysis is used to identify the best biomarker among ICU and ward patients and determine the most effective biomarker among ICU and ward patients. IgG and IgG/IgM ratios served as excellent biomarkers, followed closely by Vit E levels, the Vit E/Vit C ratio (ratio EC), and Vit D levels. Additionally, the ratios of Vit D/Vit C (DC), Vit D/Vit E (DE), and IgG/IgM (IgGIgM) are also presented in the figure.
5. Discussion
We evaluated the serum levels of vitamins C, D, and E in an Iranian cohort that had previously been vaccinated against COVID-19 but experienced disease relapse. Our findings suggest that these vitamins, either individually or in combination, significantly influence the recurrence of infection and the clinical status of the disease. Previous research has recognized the profound impact of an individual's nutritional status on the immune system, which can lead to altered immune responses, an increased risk of infection, and disease severity (15). Several clinical trials have indicated the potential benefits of micronutrient supplementation, including vitamins and minerals, in the management of COVID-19 patients (4).
Our results unequivocally demonstrate a decrease in the levels of vitamins C, D, and E among ICU patients with COVID-19. Furthermore, we observed substantial deficiencies in combinations of these vitamins among ICU patients compared to other groups (Figure 1 and Table 2). Consuming foods rich in vitamins C, D, and E, as well as minerals like zinc, can lead to a healthier lifestyle and enhanced immunity against diseases caused by bacteria, viruses, and parasites. Some studies have suggested that flavonoids may have the potential to inhibit the transmission of COVID-19.
There is a substantial body of evidence, both from animal studies and clinical trials, supporting the beneficial effects of antioxidant vitamins C and E on the immune response, involving both innate and adaptive pathways. These vitamins contribute to resistance against and treatment of both respiratory and systemic infections (16, 17). Nevertheless, the use of vitamin C in the treatment of COVID-19 patients remains a topic of controversy (18-20). Lee and Man-Fan Wan proposed the necessity of vitamin E in the proliferation of total T and T-helper cells in Asians (21). However, variations in human results may be attributed, at least in part, to pro-inflammatory cytokine gene polymorphisms (22). Vitamin E has been shown to play a crucial role in counteracting the oxidative stress induced by viral infections and in protecting against lung and liver damage (23).
The immunomodulatory effects of vitamin D and its supportive role in COVID-19 have been extensively demonstrated through various mechanisms (24-26). Sex hormones play a role in maintaining 25(OH)D levels through hormone metabolism pathways. In this context, the association of low sex hormone-binding globulin (SHBG), high luteinizing hormone, and high normal testosterone (T)/estradiol (E2) ratio with more significance in the elderly has been demonstrated (27-29). Consequently, we assessed the relationship between vitamin D levels and age in individuals above and below 50, revealing no significant correlation between vitamin D status and age.
Vitamins C and D are often prescribed together due to their combined benefits in supporting the immune system. Previous studies have underscored the importance of evaluating vitamins and trace elements, particularly vitamins C, D, and selenium, in COVID-19 patients (30). In our study, we identified a significant deficiency of vitamins C and D in patients compared to the control group, and this deficiency was proportional to the severity of the disease. Several studies have established a connection between the severity of COVID-19 and vitamin D deficiency, along with elevated levels of anti-SARS-CoV-2 IgGs. The synergistic effects of other vitamins, such as thiamine derivatives in combination with ascorbic acid, in the context of COVID-19 and other viral infections remain fully understood (31, 32). Adequate levels of vitamins C, D, and E, in conjunction with other vitamins and minerals, have been emphasized to alleviate clinical symptoms of COVID-19 (12). We observed a triple vitamin deficiency that was significantly more prevalent among ICU patients. Interestingly, there was no vitamin E deficiency detected in the control group.
Vitamins and minerals have demonstrated their association with the proper functioning of the immune system, and their vital and often synergistic effects have been well-established (6). In our study, lower levels of antioxidant vitamins, particularly vitamin E, were proportionally linked to the severity of the disease. Meta-analyses have reported varying effects of vitamin E supplementation on inflammatory cytokines, which may be attributed to different methodologies and variables of the study (11, 33). Some studies have shown an association between disease severity, higher viral spread, and cell damage in various tissues. Similarly, lower IgM levels have been observed in mildly ill patients, which could be attributed to lower viral loads (34). Accordingly, a negative correlation has been shown with IgM with time since symptom onset (35). Similarly, our results have indicated significant differences in IgM levels among the different studied groups despite all participants having a vaccination history. These observations may suggest a decline in immunity in these individuals within three months after receiving two vaccine doses.
Vaccination has proven to be a reliable strategy against infectious diseases and pandemics. A meta-analysis by Ao et al. provided strong evidence that vaccination can reduce the risk of reinfection to less than 50% compared to unvaccinated individuals, and this protection may last for up to 12 months (36). The effectiveness of vaccines, along with the milder cases of COVID-19 caused by the Omicron variant compared to previous variants, could significantly reduce the disease burden (37). A nationwide Danish study by Nielsen et al. demonstrated that vaccinated individuals had a significant level of protection against SARS-CoV-2 reinfection compared to unvaccinated individuals. Although waning immunity following vaccination was observed, particularly during the Omicron period, vaccine effectiveness persisted for up to 9 months (38). In our study, the patient groups experienced reinfection with SARS-CoV-2 within 6 months post-vaccination. Considering the predominant circulation of the Omicron variant, one of the factors contributing to reduced vaccine effectiveness could be the type of vaccine administered. However, we did not observe significant differences between the AstraZeneca and Sinopharm vaccines in terms of mean anti-SARS-CoV-2 immunoglobulin levels or the effects of vitamins A, C, and D among the different studied groups.
The beneficial effects are observed regardless of the type of vaccine. There is strong evidence indicating that neutralizing antibodies offer protection against COVID-19 (39). The practical evidence presented in this study suggests that both the immunity induced by anti-COVID-19 vaccines and natural immunity acquired after SARS-CoV-2 infection provide protection against SARS-CoV-2 infection. However, due to the limited number of samples, it is not possible to assert this with absolute certainty. Nevertheless, vaccine effectiveness studies involving various vaccines have demonstrated a wide range of protection against reinfection (40). Wajnberg et al. proposed that immune protection could be predicted by assessing neutralizing antibodies (41). Although their results are based on limited data, they suggested a similar decline in neutralizing antibodies for both vaccine-induced and naturally acquired immunity. We examined total IgG and IgM levels against SARS-CoV-2, which could be valuable due to the highly conserved non-RBD domains with nearly identical sequences among related coronaviruses (42).
5.1. Conclusions
In conclusion, vitamins C, E, and D3 play a crucial role in regulating immune activity by supporting various functions of the innate and adaptive immune systems. Inadequate levels of these vitamins can result in immune system dysfunction. The trio of vitamins assessed in this study may serve as potential biomarkers for protection against, and the severity of the disease provided that further details regarding clinical features or the disease's temporal evolution support the findings presented here.