1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus characterized by its positive polarity and single-stranded RNA structure, which was first identified in Wuhan, China, in December 2019 (1). It has been observed that the virus spreads from person to person, and the rate of transmission increased significantly in mid-January 2020, following reports from countries outside of China. The viral genome was sequenced after a nucleic acid test was conducted on a positive sample from a patient with pneumonia in Wuhan during 2019 - 2020 (2). Subsequently, studies on virus-related variants were conducted, and investigations were made into how these new variants affected clinical outcomes (3).
Severe COVID-19 is characterized by several key features, including bilateral pneumonia, acute respiratory failure (ARF), systemic inflammation, endothelial dysfunction, and coagulation activation (4, 5). Despite adequate thromboprophylaxis, it has been reported that there is an increased risk of venous thromboembolism (VTE) in COVID-19 pneumonia patients (6, 7). In terms of laboratory parameters, hematological abnormalities, such as lymphopenia, thrombocytopenia, elevated fibrinogen levels, high D-dimer levels, fibrinogen degradation products, and cytokines, like IL-6, have been identified as significant prognostic markers for mortality in COVID-19.
Numerous studies have established a direct link between systemic symptoms and hematological complications, including venous thrombosis resulting in pulmonary embolism or deep vein thrombosis and arterial thrombosis leading to myocardial infarction, strokes, or limb ischemia, all contributing to the high mortality rate associated with COVID-19 (8-10). Elevated levels of D-dimer and fibrinogen have consistently been found to be associated with increased mortality in many studies (11-13). The alterations in coagulation, inflammation, and fibrinolysis that accompany COVID-19 underscore the clinical significance of D-dimer, which serves as a biomarker for thrombosis, reflecting both fibrin formation and subsequent fibrin degradation (14).
Fibrinogen plays a crucial role in both hemostasis and thrombosis, serving as the primary structural and matrix component of blood clots. Fibrin(ogen) is necessary for the formation and growth of clots. Inadequate fibrinogen levels are a significant risk factor for bleeding (15, 16), while high fibrinogen levels increase the risk of thrombosis (17, 18).
Throughout the course of the disease, certain hematological parameters, including platelet count (PLT), lymphocyte count, hemoglobin levels, eosinophil count, and basophil count, as well as the neutrophil-lymphocyte ratio and platelet-lymphocyte ratio, have been associated with COVID-19 infection (19). The low PLT observed in COVID-19 is linked to both increased mortality and disease severity (20). It has been observed that patients with more severe disease or in poor condition tend to have lower PLT, and this is even more pronounced in those who do not survive. The mechanisms contributing to low platelet counts include direct viral infection of the bone marrow, platelet destruction by the immune system, platelet accumulation in the lungs, microthrombus formation, and excessive virus consumption, which may reduce platelet synthesis by interacting with megakaryocytes (21).
2. Objectives
The aim of our study was to investigate the variations in D-dimer, fibrinogen, PLT, and mean platelet volume (MPV) among the Alpha, Beta, Delta, and Omicron variants.
3. Methods
3.1. Patients
The study retrospectively evaluated the D-dimer, fibrinogen, PLT, MPV, and SARS-CoV-2 mutation levels in SARS-CoV-2 positive patients who presented to our hospital between 2020 and 2023. A total of 28 195 patients were included in the study.
3.2. Inclusion and Exclusion Criteria
Patients who tested positive for SARS-CoV-2 reverse transcription (RT)-PCR were included, while suspicious and negative samples were excluded from the study. The study included adult individuals aged 18 and above but excluded pediatric patients. Data from patients with an unknown SARS-CoV-2 variant status were also excluded from the study.
3.3. SARS-CoV-2 Analysis
3.3.1. Variant and Test Analysis
The study was conducted in our hospital's laboratory using RT-PCR kits (Bio-Rad Laboratories, USA) to detect SARS-CoV-2 in nasopharyngeal samples from patients. SARS-CoV-2 Alpha and Beta variants were identified using the Bio-Speedy® SARS-CoV-2 Variant Plus Kit (Bioeksen AR-GE Technologies, Turkey), which targets ORF1ab and N gene regions as well as variant-specific genome regions found only in B.1.1.7, B.1.351, and P.1 (RNAase P internal quality control, SE484K Beta/gama, N31-33, and SL452R Delta). The CFX96 DX Real-Time PCR systems (Bio-Rad Laboratories, USA) were employed for this purpose. SARS-CoV-2 Delta and Omicron variants were analyzed through 16S ribosomal RNA (rRNA) using the Illumina Miseq sequencing technique. Serum and blood samples from the patients were analyzed for D-dimer, PLT, and MPV values using Cobas 800 (Roche) devices, while fibrinogen levels were measured by the Stago STA-Compact-Max analyzer. All data were recorded.
3.4. Statistical Analysis
In accordance with the test groups, data for D-dimer (n = 7090), fibrinogen (n = 5709), PLT (n = 7066), and MPV (n = 8330) were analyzed by segregating them based on variant groups. The suitability of the data for normal distribution was assessed using the Shapiro-Wilks test and Box Plot graphics. To compare normally distributed quantitative data between groups, the One-way ANOVA was employed, and the Bonferroni test was utilized to identify the group responsible for any differences. Qualitative data were compared using Fisher's exact test. The results were assessed at a 95% confidence interval, with a significance level set at P < 0.05.
4. Results
Regarding gender in all test groups, the incidence of the delta variant was higher in women compared to the other variants, with alpha and omicron variant patients following closely (P = 0.001). The beta variant was found at a higher rate in male patients. There was a significant difference in variants based on age (P = 0.001; P < 0.01); the ages of cases with the omicron variant were higher than those with alpha, beta, and delta variants (P = 0.001, P = 0.001, P = 0.001, and P < 0.01, respectively). Additionally, the ages of cases with delta variants were higher than those with beta variants (P = 0.004 and P < 0.01, respectively) (Table 1).
| Variables | Alpha (n = 2840) | Beta (n = 1576) | Delta (n = 2114) | Omicron (n = 1800) | P-Value | Post-Hoc Results b |
|---|---|---|---|---|---|---|
| Gender | 0.001 c | 4 > 1, 2, 3; 3 > 1, 2; 1 > 2 | ||||
| Female | 1453 (51.2) | 747 (47.4) | 1245 (58.9) | 911 (50.6) | ||
| Male | 1387 (48.8) | 829 (52.6) | 869 (41.1) | 889 (49.4) | ||
| Age | 0.001 d | |||||
| Mean ± standard deviation | 52.68 ± 17.63 | 52.08 ± 15.83 | 54.74 ± 19.13 | 59.84 ± 20.40 | ||
| Median (min - max) | 52.08 (18 - 95) | 52.15 (18 - 94) | 54.61 (18 - 93) | 64.23 (18 - 99) |
a Values are expressed as No. (%), unless otherwise indicated.
b Alpha, beta, delta, and omicron groups are considered as 1, 2, 3, and 4, respectively.
c Fisher-Freeman-Halton test.
d One-way ANOVA.
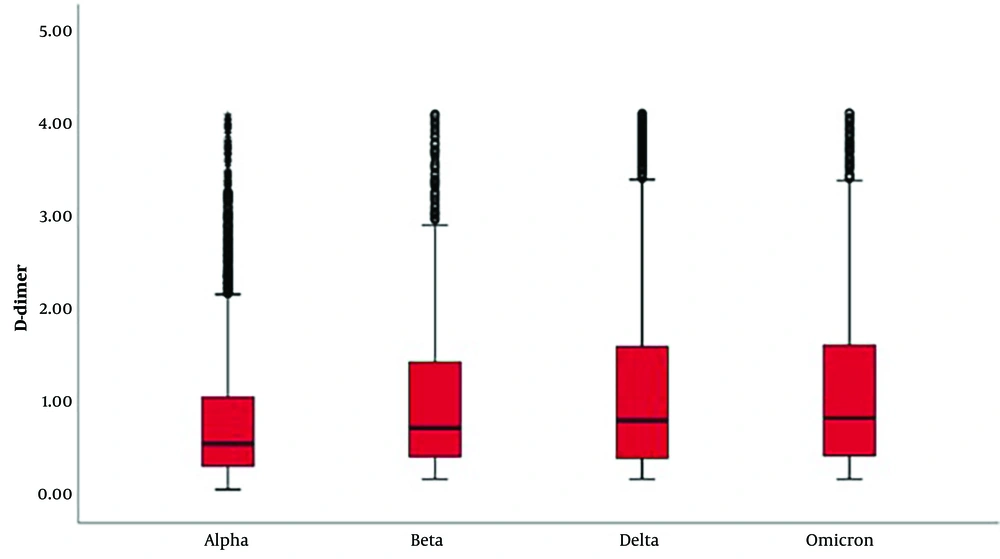
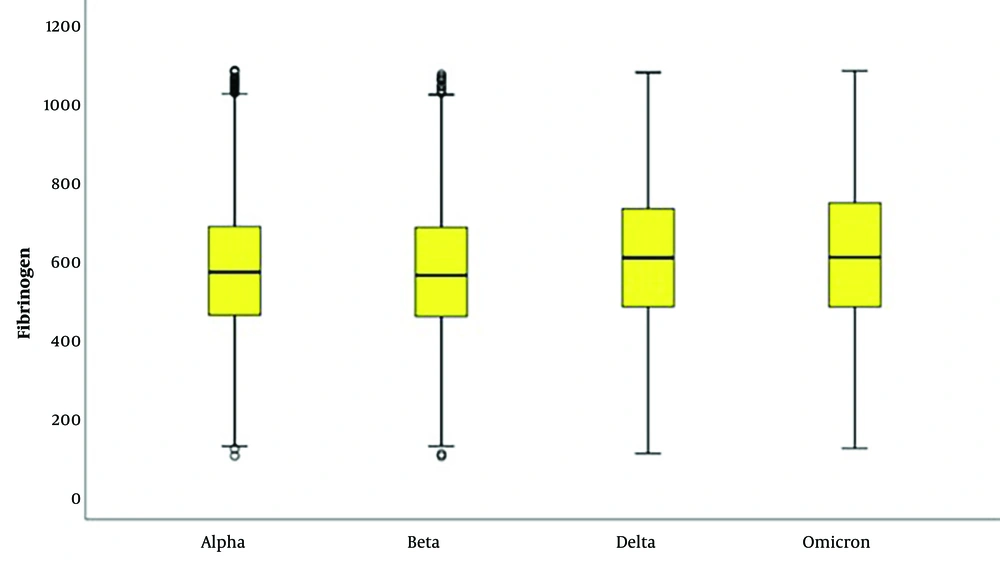
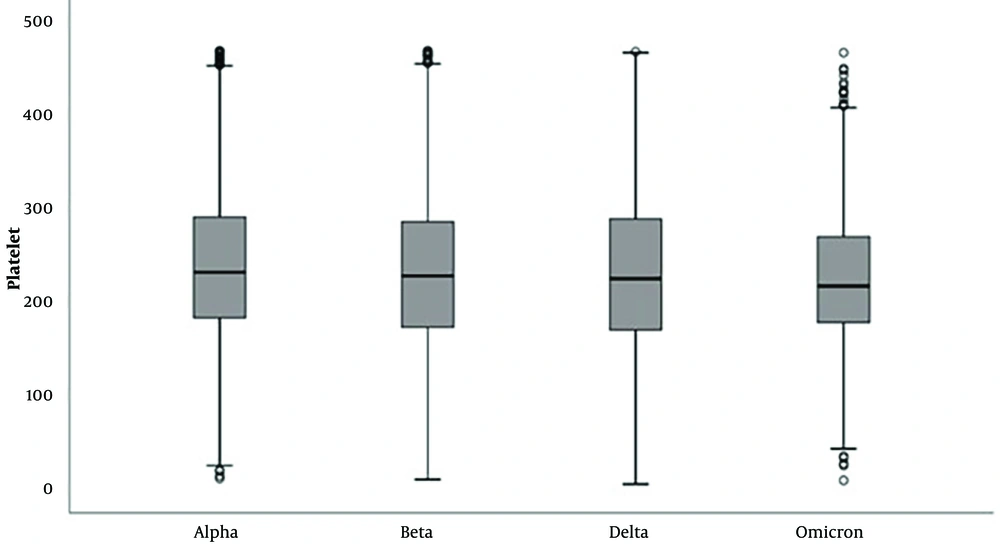
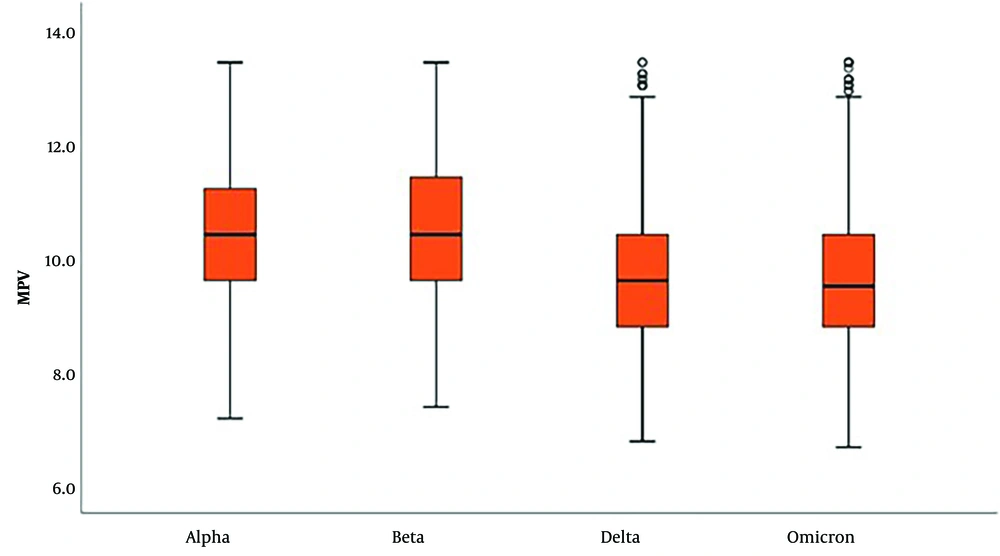
In laboratory parameters, D-dimer and fibrinogen levels were significantly higher in delta and omicron variants (Tables 2 and 3; Figures 1 and 2). PLT and MPV were found to be lower in SARS-CoV-2 Delta and Omicron variants compared to Alpha and Beta variants (Tables 4 and 5; Figures 3 and 4).
| D-Dimer Levels (µg/mL) | Alpha (n = 2755) | Beta (n =1324) | Delta (n = 1652) | Omicron (n = 1359) | P-Value | Post-Hoc Results b |
|---|---|---|---|---|---|---|
| Normal | 1217 (44.2) | 416 (31.4) | 526 (31.8) | 402 (29.6) | 0.001 c | 4 > 1, 2; 3; > 1, 2; 2 > 1 |
| High | 1538 (55.8) | 908 (68.6) | 1126 (68.2) | 957 (70.4) | ||
| Mean ± standard deviation | 0.85 ± 0.78 | 1.07 ± 0.89 | 1.17 ± 1.01 | 1.18 ± 0.98 | 0.001 d | 1 < 2, 3, 4 |
| Median (min-max) | 0.57 (0.08 - 4.9) | 0.74 (0.1 - 4.1) | 0.82 (0.1 - 4.1) | 0.85 (0.1 - 4.1) |
a Values are expressed as No. (%), unless otherwise indicated.
b Alpha, beta, delta, and omicron groups are considered as 1, 2, 3, and 4, respectively.
c Fisher-Freeman-Halton test.
d One-way ANOVA.
| Fibrinogen Results (mg/dL) | Alpha (n = 2712) | Beta (n = 851) | Delta (n = 1183) | Omicron (n = 963) | P | Post-Hoc Results b |
|---|---|---|---|---|---|---|
| Mean ± standard deviation | 576.90 ± 176.21 | 570.06 ± 183.26 | 612.72 ± 194.11 | 621.56 ± 197.96 | 0.001 c | 4 > 1, 2; 3 > 1, 2 |
| Median (min - max) | 567 (96 - 1084) | 559 (95 - 1074) | 604 (102 - 1079) | 605 (115 - 1084) | ||
| Low | 41 (1.5) | 18 (2.1) | 11 (0.9) | 5 (0.5) | 0.032 d | 4 < 1, 2; 2 > 3 |
| Normal | 388 (14.3) | 119 (14.0) | 158 (13.4) | 120 (12.5) | ||
| High | 2283 (84.2) | 714 (83.9) | 1014 (85.7) | 838 (87.0) | 4 > 1 |
a Values are expressed as No. (%), unless otherwise indicated.
b Alpha, beta, delta, and omicron groups are considered as 1, 2, 3, and 4, respectively.
c One-Way ANOVA test (P < 0.01).
d Fisher Freeman Halton test (P < 0.05).
| Platelet Count Results, 103/µL | Alpha (n = 2770) | Beta (n = 1530) | Delta (n = 1752) | Omicron (n = 1014) | P-Value | Post-Hoc Results b |
|---|---|---|---|---|---|---|
| Mean ± standard deviation | 238.92 ± 80.66 | 229.42 ± 89.10 | 230.15 ± 93.39 | 223.78 ± 73.27 | 0.001 c | 1 > 2, 3, 4 |
| Median (min - max) | 230 (9 - 467) | 226 (8 - 467) | 223 (3 - 466) | 215 (7 - 465) | ||
| Low | 321 (11.6) | 261 (17.1) | 128 (7.3) | 27 (2.7) | 0.001 d | 1 > 3, 4; 2 > 1, 3, 4; 3 > 4 |
| Normal | 2327 (84.0) | 1200 (78.4) | 1527 (87.2) | 966 (95.3) | 2 < 1, 3, 4; 1 < 3,4; 4 > 3 | |
| High | 122 (4.4) | 69 (4.5) | 97 (5.5) | 21 (2.1) | 4 < 1, 2, 3 |
a Values are expressed as No. (%), unless otherwise indicated.
b Alpha, beta, delta, and omicron groups are considered as 1, 2, 3, and 4, respectively.
c One-way ANOVA.
d Fisher-Freeman-Halton test.
| MPV Results, fL | Alpha (n = 2840) | Beta (n = 1576) | Delta (n = 2114) | Omicron (n = 1800) | P-Value | Post-Hoc Results b |
|---|---|---|---|---|---|---|
| Mean ± standard deviation | 10.40 ± 1.13 | 10.50 ± 1.21 | 9.65 ± 1,15 | 9.63 ± 1.18 | 0.001 c | 2 > 1, 3, 4 |
| Median (min - max) | 10.4 (7.2 - 13.4) | 10.4 (7.4 - 13.4) | 9.6 (6.8 - 13.4) | 9,5 (6.7 - 13.4) | 1 > 3, 4 | |
| Low | 430 (15.1) | 6 (0.4) | 47 (2.2) | 71 (3.9) | 0.001 d | 1 > 2, 3, 4; 2 < 3, 4 |
| Normal | 2164 (76.2) | 1545 (98.0) | 2060 (97.4) | 1718 (95.4) | 1 < 2, 3, 4; 4 < 2, 3 | |
| High | 246 (8.7) | 25 (1.6) | 7 (0.3) | 11 (0.6) | 1 > 2, 3, 4; 2 > 3, 4 |
a Values are expressed as No. (%), unless otherwise indicated.
b Alpha, beta, delta, and omicron groups are considered as 1, 2, 3, and 4, respectively.
c One-way ANOVA.
d Fisher-Freeman-Halton test.
5. Discussion
COVID-19 primarily affects middle-aged and elderly individuals (22), with children and young people often experiencing the illness asymptomatically. In our study, we observed an increase in the average age from the alpha variant to the omicron variant. Regarding gender distribution, the delta variant appears to be more prevalent in females, while the beta variant is more common among males. This observation may be attributed to quarantine measures implemented since the beginning of the pandemic, which could have delayed virus exposure among women and the elderly.
COVID-19 primarily manifests as a respiratory disease with symptoms resembling viral pneumonia, but it also impacts other organ systems such as the heart, kidneys, and brain. Existing research suggests that the activation of the hemostatic system significantly influences the pathological manifestations of SARS-CoV-2 infection. Fibrinogen, a clotting protein, is one of the most abundant plasma proteins.
Current findings indicate that elevated levels of fibrinogen and the fibrin degradation product D-dimer serve as biomarkers for poor prognosis in COVID-19 (23). Pulmonary microvascular thrombosis has been documented, and the risk of arterial thrombotic disease has increased, although bleeding events are less common than thrombotic events. In our study, when D-dimer results were analyzed according to variants, we observed an increase in levels from the alpha to the omicron variant. It is known that elevated D-dimer levels are associated with increased mortality.
Studies have demonstrated that as the virus has evolved, transmission rates have risen while mortality rates have declined. Compared to the wild-type SARS-CoV-2 and the alpha (B.1.1.7), beta (1.351), and delta variants, SARS-CoV-2 omicron infection has resulted in the lowest reduction in body weight and the lowest mortality rate (24). This paradox is quite striking. In another study, D-dimer levels in the omicron variant were found to be lower when compared to the delta variant. Additionally, advanced age and male gender have been identified as factors contributing to increased D-dimer levels (25).
Risk factors for arterial thrombosis include older age, male gender, Hispanic ethnicity, a history of coronary artery disease, and D-dimer levels exceeding 230 ng/mL upon presentation (26). It is possible that in our study, the higher detection of D-dimer levels in the omicron variant could be attributed to a higher average age. Additionally, the higher number of females in the delta variant might have resulted in lower D-dimer levels compared to omicron. The reported incidence of thrombotic complications in COVID-19 varies between 7% and 60% (27, 28). In our study, a significant increase in D-dimer and fibrinogen levels was observed from the alpha to omicron variants. Deep vein thrombosis and pulmonary embolism are the most frequently reported thrombotic events in SARS-CoV-2 infection (29, 30), but some studies suggest that 58% of COVID-19 deaths are associated with arterial thrombosis (31). Microvascular thrombosis, particularly in the lungs, has been reported in 57% of COVID-19 patients; this rate is considerably higher than the 24% rate reported for influenza A patients (32).
5.1. Conclusions
In our study, we observed that platelet levels and MPV values were lower in the delta and omicron variants compared to the alpha and beta variants. The change in coagulation parameters from the alpha to the omicron variant suggests an increased tendency to clot. This observation leads us to consider that the disease could potentially be more fatal in the absence of vaccination and herd immunity.