1. Background
Fungal infections substantially threaten public health, particularly in developing countries, where they significantly contribute to mortality rates (1, 2). Among pathogenic fungi, Candida albicans is a formidable agent responsible for various manifestations of candidiasis (1). It exhibits a propensity to form biofilms on diverse surfaces, including medical devices and human epithelial linings, presenting challenges in treatment (3-5). This fungus is a leading cause of oral infections, affecting the mucous membranes of the mouth and oropharynx. The prevalence of oral candidiasis is notably higher in vulnerable populations such as geriatric and immunocompromised patients. It is known for resisting conventional antifungal agents like amphotericin B and fluconazole (4, 5).
In the pharmacotherapeutic landscape, the gold standard for treating oral candidiasis is nystatin mouthwash, a fungicidal drug with a mechanism of action that alters the permeability of fungal membranes by binding to membrane sterols (6). However, the drawbacks associated with nystatin, including a bitter taste, frequent administration (four times a day), and the specific preparation method, contribute to patient dissatisfaction (7, 8). These limitations underscore the pressing need for innovative treatment modalities to address the challenges posed by existing therapies.
Cold atmospheric plasma (CAP) has demonstrated versatility in dentistry, with applications ranging from deactivating oral pathogens to treating periodontitis, wound repair, and teeth bleaching (9-11). The underlying mechanism of CAP involves the generation of active oxygen and nitrogen, complemented by electric fields, heat, and radiation, collectively reducing the microbial load in the target area (12-14).
CAP has different subtypes with unique generation methods and applications (15-17). Non-thermal atmospheric plasma can treat onychomycosis and eradicate C. albicans and Trichophyton mentagrophytes in 12 minutes (15). A 10 - 15 minute plasma jet exposure can stop mycelial growth with a single exposure (16). Cold atmospheric microwave plasma can be a novel antifungal solution against Malassezia pachydermatis, and it has a synergistic antifungal effect when combined with chlorhexidine gluconate (17). Dielectric barrier discharge (DBD) plasma is one of the CAP subtypes being studied to eradicate microorganisms, including fungal infections in agriculture and disinfecting seeds (18-20). Wu et al. (19) showed that a 9-minute treatment with DBD plasma significantly reduces the spore germination rate (95.48%) and spore viability (98.82%) of Colletotrichum asianum. Dielectric barrier discharge plasma treatment also effectively reduces the microbial population on the surface of ginseng seeds, which helps mitigate root rot (20).
2. Objectives
Regrettably, there are insufficient studies analyzing the antifungal potential of DBD plasma applications in human candida species. Given the increasing prevalence of drug-resistant strains, especially the common antifungal drug-resistant C. albicans, and the limited number of investigations into the effects of DBD on the oral strain of C. albicans, the need for this study has become evident. This study aims to comprehensively investigate the effects of DBD plasma radiation on C. albicans, a prevalent oral fungal pathogen. The investigation will be conducted under carefully controlled laboratory conditions to gain a deeper understanding of this radiation's potential impact and applications on the targeted microorganism.
3. Methods
3.1. Sample Size
The sample size of this study was determined to be eight in each subgroup using the one-way ANOVA power analysis option in PASS 11 software (α = 0.05, effect size = 0.51, β = 0.2, and mean standard deviation = 12%). Three times CAP irradiations were performed for each strain. The total sample size was 226 (9 × 3 × 8 + 10), considering eight sensitive and one standard strain of C. albicans, one positive, and one negative control group.
3.2. Preparation of Candida albicans Strain Suspension
The standard strain of C. albicans (ATCC 10231) was obtained from the American Type Culture Collection. Eight clinical C. albicans strains were collected from the inner surface of dentures belonging to individuals diagnosed with denture stomatitis who had been subjects of prior research initiatives. These specimens were archived and accessible in the repository of the Department of Medical Mycology in Iran. The yeast was subcultured from vial stocks onto Sabouraud dextrose agar (SDA; Merck, Germany) under aerobic conditions at 37°C. Then, a pure colony on the SDA medium was isolated using a glass loop and transferred to a tube containing 2 mL of physiological saline solution (0.9%). A suspension was prepared with a density of 0.5 McFarland. The turbidity of the cell suspensions was measured using a spectrophotometer at 540 nm to achieve a concentration equivalent to 1.5 × 108 CFU / mL. This suspension was used for the DBD plasma treatment and MTT (3,4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay.
3.3. Dielectric Barrier Discharge Plasma Device
This study utilized a DBD device (Sepid Jamegan Fan Avran, Iran). The device's power was set at 100 KHz and operated with the HVRF power input of 8kW. To generate plasma, neon gas was injected into the device with a flow rate of 4 L/min. For plasma treatment, 100 µL of the C. albicans suspension was poured into a 96-well plate and divided into 8 groups (Table 1). Then 64 µg/mL nystatin (NYS; Sigma-Aldrich, USA) was added in some groups. Then, the wells of the plate were subjected to DBD plasma for 5, 10, and 15 minutes at a distance of 2 mm from the surface of the culture medium.
| No. | Groups |
|---|---|
| 1 | 5-min DBD plasma irradiation |
| 2 | The combination of nystatin and 5-min DBD plasma irradiation |
| 3 | 10-min DBD plasma irradiation |
| 4 | The combination of nystatin and 10-min DBD plasma irradiation |
| 5 | 15-min DBD plasma irradiation |
| 6 | The combination of nystatin and 15-min DBD plasma irradiation |
| 7 | Positive control (Candida albicans and 64 µg/mL nystatin without DBD plasma irradiation) |
| 8 | Negative control (C. albicans without DBD plasma irradiation) |
3.4. Measurement of Candida albicans Viability
3.4.1. Colony Numbers
After DBD plasma treatment, approximately 100 µL of the C. albicans irradiated suspension (1.5 × 108 CFU/mL) were seeded on SDA plates. Using a glass spreader, the cells were evenly distributed on the plates. The plates were then incubated at 37°C for 24 hours, and the number of colonies on each plate was counted. This process was repeated 8 times, and the mean number of colonies was reported.
3.4.2. MTT Colorimetric Assay
Following the exposure period (5, 10, and 15 minutes), 100 μL of the suspensions per well were transferred to a 96-well plate. Subsequently, 15 μL of MTT (Sigma-Aldrich, USA) was added to each well at a concentration of 5 mg/mL in water. The plate was then incubated at room temperature for 3 hours. The contents in each well were transferred to 1.5 mL microcentrifuge tubes, which were then centrifuged for 2 minutes at 10,000 rpm. After removal of the liquid, cells were washed once with 100 μL of water. Then, 70 μL of dimethyl sulfoxide (DMSO) was added to each tube. The tubes were incubated at room temperature for 3 minutes, and then the contents in each tube were transferred to a new 96-well plate. The plate was read at 540 nm using an ELISA reader device (Anthos company, Austria).
3.5. Statistical Analysis
The data were analyzed using SPSS version 26. The Kolmogorov-Smirnov test was used to evaluate the normal distribution of data, and one-way ANOVA, two-way ANOVA, independent sample t-test, Tukey test, and Tamhane were used to assess the number of colonies and cell viability.
4. Results
4.1. Evaluation of Candida albicans Viability
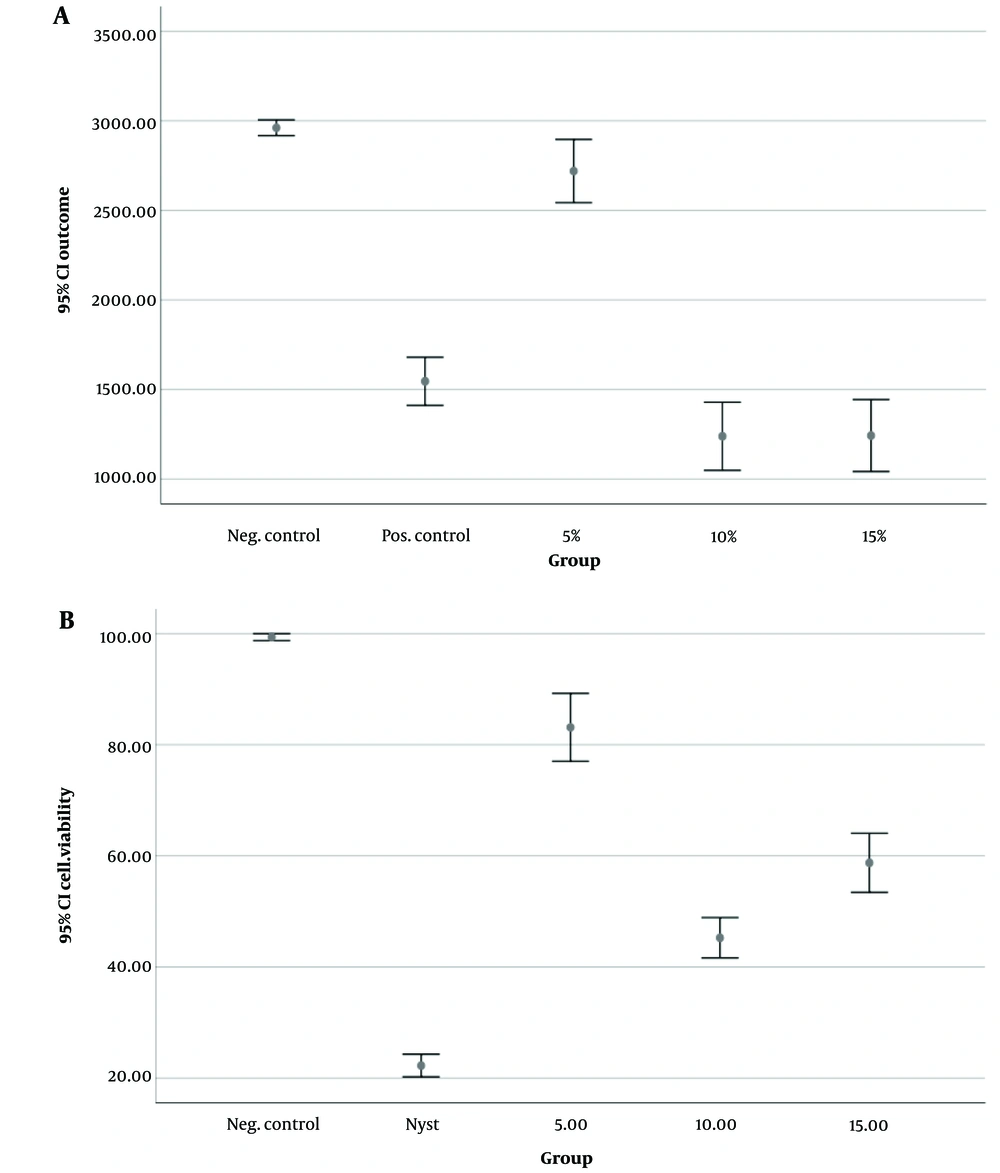
Figure 1 shows the results of C. albicans cell viability evaluation using colony count (Figure 1A) and MTT assay (Figure 1B).
4.1.1. Colony Numbers
The results showed the lowest mean number of C. albicans colonies after the 10-minute DBD plasma irradiation (1238.88 ± 247.52), followed by the 15-minute DBD plasma irradiation (1243.05 ± 261.83). The negative control group, without plasma irradiation, exhibited the highest mean number (2961.11 ± 57.43). Based on the one-way ANOVA, a significant difference was found among the studied groups regarding the mean number of C. albicans colonies (P < 0.001).
4.1.2. MTT Colorimetric Assay
The data indicates that the lowest cell viability occurred following the application of nystatin, followed by a 10 minute exposure to DBD plasma. In groups without nystatin, all plasma irradiation periods resulted in significantly lower cell viability compared to the control group (no irradiation) (P < 0.001). A 10-minute plasma exposure led to significantly fewer viable cells than both 15- and 5-minute exposures (P < 0.001), and 15-minute plasma irradiation resulted in fewer viable cells than 5 minutes (P < 0.001). In the nystatin group, while the mean cell viability of C. albicans in all plasma irradiation groups significantly decreased compared to the control group (P < 0.001), there were no significant differences among the 10-, 15-, and 5-minute plasma irradiation groups (P > 0.05).
5. Discussion
Oral candidiasis, characterized by opportunistic infection, is highly prevalent among specific demographics such as children, the elderly, and immunocompromised individuals (21, 22). Recently, there has been a rise in reports of antifungal-resistant oropharyngeal candidiasis cases (2, 23, 24), posing significant challenges due to the limited availability of new treatments and medications (25). A potential alternative treatment for candidiasis, specifically in the case of oral infections, could be the use of CAP (1, 24, 26, 27). Wang et al. showed that cold plasma is effective against oral C. albicans infection (26). Fricke found that plasma treatment can rapidly remove C. albicans biofilm, especially when added oxygen (28). Yamazaki et al. showed that low-frequency atmospheric pressure plasma jets could be a solution in clinical dentistry for preventing C. albicans-associated denture stomatitis (29). Also, Avukat found that helium plasma treatment can effectively prevent denture stomatitis caused by C. albicans (27).
However, a standard protocol for CAP application has yet to be established. Prior studies have explored varying irradiation times, power levels, and plasma types, yielding conflicting outcomes (1, 5, 30). For instance, Wanachantararak et al. (30) demonstrated that 15 minute argon DBD plasma irradiation at 20W was more effective against C. albicans than in 5- and 10-minute durations. On the other hand, Ebrahimi-Shaghaghi et al. (5) indicated that a 180-second cold plasma irradiation using helium was more beneficial, and Leite found 5-minute DBD plasma irradiation using helium with 13-watt power to be more effective (1). Matthes et al. (18) revealed that volume DBD (VDBD) could help reduce denture-associated candidiasis for newer biofilms, but older biofilms needed mechanical pre-treatment for better disinfection. He found that VDBD argon plasma is effective against 2-day-old candida biofilms but not against 7 or 16-day-old biofilms, and its combination with chlorhexidine gluconate or sodium hypochlorite cannot enhance antimicrobial effects (18).
In this study, the effectiveness of neon DBD plasma irradiation for 5, 10, and 15 minutes at 8 kW power was evaluated to determine C. albicans viability and colony counts in 2 groups, with and without nystatin, using MTT. The MTT assay is a colorimetric test that measures cell metabolic activity by assessing mitochondrial activity. It is a simple and easy method for analyzing cell viability. The test relies on the conversion of yellow tetrazole MTT dye to purple formazan by living cells. The intensity of the produced color is measured by spectrophotometric methods at 570 nm and is proportional to the number of living cells. During the MTT assay, DMSO should be added to the wells containing examining cells because this step is essential for extracting the color from the formazan crystals, rendering it measurable. DMSO is an organic solvent capable of dissolving formazan crystals produced by viable cells (31). The results of our study indicated that the combined treatment of nystatin and DBD plasma irradiation was the most effective against C. albicans.
Cold atmospheric plasma functions by inhibiting cell membrane activity and inducing significant changes in permeability, enhancing the sensitivity of fungi and bacteria to antifungal agents like polyene antifungals (32, 33). Consequently, it synergizes with the effects of nystatin, causing substantial morphological alterations in C. albicans' structure and triggering ROS-induced apoptosis, leading to the oxidation of intracellular organelles (5). Laboratory studies have also reported CAP's inhibitory impact on adhesion, filamentation, and ergosterol synthesis of C. albicans (5, 34). The effectiveness of plasma treatment relies on producing active oxygen and nitrogen, supported by the electric field, heat, and radiation (12). Notably, the application of surface plasma can induce genetic modifications in C. albicans' genome, impacting the nuclear genome and reducing hydrolytic enzyme activity (2). While plasma treatment can decrease the viability and colony numbers of C. albicans, complete eradication within a short irradiation time is not achievable (2). In this study, both groups with and without nystatin showed that 15- and 10-minute plasma irradiation was more effective than 5 minute irradiation.
It's important to acknowledge that the combined effects of antifungal drugs can vary based on the strains examined and the types of medications used (35). In this study, applying DBD plasma for 10 minutes exhibited the most potent antifungal effects against C. albicans. However, sole DBD plasma irradiation was less effective than conventional nystatin treatment. Contrastingly, Leite et al.s’ research suggested that 5 minute DBD cold plasma irradiation, either with nystatin or amphotericin B, did not synergize with C. albicans biofilm and, in fact, found sole cold plasma radiation to be more effective than its combination with antifungal medications (1). These divergent outcomes could stem from using different plasma types and strains.
Despite the strength of the present study, there were several limitations as well. Since none of our candida strains was nystatin-resistant, we could not analyze the effects of DBD plasma irradiation on the resistant C. albicans. In addition, it was observed that different strains responded differently to the DBD plasma treatment. These variations in each strain's response to the treatment procedures could be attributed to genetic differences. However, we did not analyze each strain's genotype and genomic charts. Therefore, we recommend future studies to focus on this aspect. As our research is primarily focused on the C. albicans cells in an in vitro condition, we encounter limitations in assessing both the positive and adverse events associated with the application of DBD plasma in animal and human cell subjects. Consequently, we recommend the implementation of comprehensive animal and clinical trial studies to provide a more accurate understanding of the effects of DBD plasma irradiation.
5.1. Conclusions
This study showed that the sole irradiation of DBD plasma was not as effective as the conventional nystatin treatment. The DBD plasma irradiation could synergize the antifungal effects of nystatin, and 10-minute plasma irradiation accompanied by nystatin treatment is more effective than in the other groups.