1. Background
Invasive aspergillosis (IA) poses a significant threat to hematology departments, primarily affecting patients with hematologic malignancies (HM) who have undergone chemotherapy and are in a neutropenic status (1). Among HM patients, IA manifests primarily in the lungs and stands as the most severe form of aspergillosis (2). A prior study conducted in Iran reported an incidence rate of 23.33% for IA in HM patients (3). Hematopoietic stem-cell transplantation (HSCT), acute leukemia, myelodysplastic syndrome (MDS), and prolonged neutropenia were identified as the primary predisposing factors for IA (3, 4). The mortality rate associated with IA in acute leukemia patients ranges from 70% to 90% (1, 3, 4).
Aspergillus fumigatus is the most common causative agent of IA. The infection occurs through the inhalation of Aspergillus conidia, which are widely distributed in the environment (5). IA can affect various organs, including the lungs, brain, spine, heart, ear, eye, and skin (3). However, several cancer centers have recently reported a significant rise in the incidence of IA caused by non-A. fumigatus species, which are uncommon and resistant to treatment (6-8). In patients with HM, the gold standard for diagnosing IA is histopathological evaluation through tissue biopsy. However, this may not be feasible due to the risk of severe thrombocytopenia (9). Consequently, promptly diagnosing IA can be highly challenging. While there may be signs and symptoms suggestive of invasive pulmonary aspergillosis (IPA), distinguishing between IPA and other pulmonary mycoses remains exceptionally difficult (10).
2. Objectives
Considering the existing diagnostic and therapeutic challenges in managing IA patients, conducting a comprehensive evaluation of these patients can enhance our understanding of therapeutics and potential changes in clinical characteristics, radiological manifestations, and patient management. In this study, we aimed to determine the incidence of IA in patients with HM and provide a detailed description of these patients, including their clinical symptoms, risk factors, radiological findings, diagnostic methods, antifungal treatment, and treatment responses. This investigation was conducted in the hematology department of a tertiary clinical center in Tehran, Iran, between 2018 and 2022.
3. Methods
3.1. Study Design and Setting
We conducted a retrospective study on patients with HM who were diagnosed with IA during their hospitalization at Imam Khomeini Hospital Complex, Tehran, Iran, from March 2018 to March 2022.
3.2. Study Population
The study focused on patients with HM who were diagnosed with probable or proven IA. Adult patients with leukemia, lymphoma, and multiple myeloma, whose IA diagnosis was based on the 2019 definition of invasive fungal infections (IFIs) by the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG) (11), were included in the study. Patients who had undergone HSCT or solid organ transplantation (SOT), those with solid tumors, COVID-19, a history of IFIs, or those with ambiguous or incomplete information were excluded.
According to the local therapeutic protocol for HM patients undergoing chemotherapy, fluconazole (400 mg/PO/daily) was prescribed at the beginning of chemotherapy as antifungal prophylaxis. When there was suspicion of an invasive fungal infection or when a patient with fever and neutropenia did not respond to antibiotic treatment after 3 - 5 days, a serum galactomannan (GM) test was conducted, and paranasal sinuses (PNS) and chest CT scan were requested. Additionally, liposomal amphotericin B or caspofungin was immediately prescribed. If the CT scan indicated lung or sinus involvement consistent with an invasive fungal infection, endoscopy was considered if feasible.
3.3. Data Collection and Laboratory Tests
We extracted patients' demographic and clinical characteristics, including the length of hospital stay, underlying diseases, mycological features, antifungal and antibiotic regimens, response to therapy, all-cause mortality, and other outcomes, from the hospital information system (HIS) database. Radiological images of the chest and PNS were evaluated by a radiologist. Samples were previously examined directly using 10% potassium hydroxide (KOH) and cultured on Sabouraud's dextrose agar (SDA) with 0.5 mg/ml chloramphenicol (Merck, Germany) and incubated at 30°C for one week. Differential media such as potato dextrose agar (PDA, Merck, Germany) and Czapek agar (CZ, Micro media, Hungary) were employed to initially identify isolated Aspergillus species based on colony appearance and microscopic characteristics of vegetative hyphae, conidial heads, phialides, vesicle, and conidiophore using lactophenol cotton blue staining. To confirm identified species, all isolates underwent polymerase chain reaction (PCR) and sequencing techniques (3). The Platelia Aspergillus GM EIA kit (Bio-Rad, France) was used to measure the GM antigen level in serum and bronchoalveolar lavage (BAL) samples via the ELISA method following the manufacturer's instructions (3, 10). All GM positive results were double-checked before being considered positive.
3.4. Statistical Analysis
We conducted the statistical analysis using SPSS version 26. The data were summarized using descriptive statistics, with continuous variables presented as either median and interquartile range or mean and standard deviation, and categorical variables represented as proportions or percentages. All statistical tests were reported at a 95% confidence level, with a significance level set at a p-value of less than 0.05.
4. Results
4.1. Study Population and Baseline Characteristics
A total of 393 patients who were hospitalized in the hematology ward of Imam Khomeini Hospital Complex were initially evaluated, out of which 350 patients had HM and were considered for the study. Ultimately, data from 51 patients with IA (14.57%) who met the eligibility criteria were included in the analysis. Detailed patient characteristics can be found in Table 1. The average age of the patients was 36.7 years (range: 14 - 78 years). The highest prevalence of IA was observed in the age group of < 50 years, with 41 cases (80.4%). Overall, the incidence of IA was higher in males (n = 34) than in females (n = 17), occurring twice as often. The mean length of hospital stay was 104.9 days (range: 6 - 221). According to the revised and updated (2019) definitions for IFIs by EORTC/MSG (9), out of the 51 cases of aspergillosis in this study, 11 cases (21.6%) were proven, and 40 cases (78.4%) were classified as probable.
| Variables and Categories | No. (%) |
|---|---|
| Age, y | |
| < 50 | 41/51 (80.4) |
| ≥ 50 | 10/51 (19.6) |
| Gender | |
| Female | 17/51 (33.3) |
| Male | 34/51 (66.7) |
| Hematologic malignancy | |
| AML | 28/51 (54.9) |
| ALL | 16/51 (31.4) |
| Lymphoma | 7/51 (13.7) |
| CT findings | |
| Halo sign | 31/51 (72.1) |
| Nodules | 12/51 (27.9) |
| Cavitary lesion | 8/51 (18.6) |
| Wedge shape lesion | 7/51 (16.3) |
| Air crescent sign | 4/51 (9.3) |
| PNS involvement | 35/45 (77.8) |
| Nasal septum destruction | 7/45 (20) |
| Orbital invasion | 3/45 (8.6) |
| Aspergillus species | |
| Flavus | 19/24 (79.2) |
| Niger | 3/24 (12.5) |
| Others | 2/24 (8.3) |
| EORTC/MSG definitions | |
| Probable | 40/51 (78.4) |
| proven | 11/51 (21.6) |
| Death | |
| AML | 5/8 (62.5) |
| ALL | 2/8 (25) |
| Lymphoma | 1/8 (12.5) |
Abbreviations: ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; PNS, para nasal sinuses.
4.2. Diagnostic Tests
The results of diagnostic tests conducted on several patients revealed that among the 51 patients diagnosed with IA, 5 (9.8%) had positive results in direct examination and culture of BAL samples, and 19 (37.3%) showed positive results in direct examination and culture of sinus samples. The most frequently isolated species was A. flavus (n = 19, 79.2%), followed by A. niger (n = 3, 12.5%). Additionally, 2 patients (3.9%) had positive histopathology results for lung samples, while 9 (17.6%) had positive sinus histopathology.
The results of PCR and sequencing were consistent with the macroscopic and microscopic identifications of the isolates. The GM antigen, a major constituent shed from Aspergillus cell walls during aspergillosis, was detected in serum and BAL samples of all patients, with mean levels of 0.997 and 1.95 ng/dL, respectively. A GM antigen level of ≥ 1 ng/dL was considered positive (10). Consequently, 25 patients (49%) had positive GM antigen levels in either serum or BAL samples. The concordance of culture results with the GM test was 64% (16/25).
4.3. Hematologic Malignancies and Underlying Diseases
Among the 51 patients with IA and HM, 28 (54.9%) had acute myeloid leukemia (AML), 16 (31.4%) had acute lymphoblastic leukemia (ALL), and 7 (13.7%) had lymphoma (refer to Table 1). It is noteworthy that out of these patients, 40 (78.4%) had no history of underlying diseases, while 9 (17.6%) had a history of other hematological disorders (including thalassemia minor, myelodysplastic syndromes (MDS), Coombs-negative hemolytic anemia, immune thrombocytopenia (ITP), megaloblastic anemia, and iron deficiency anemia). Additionally, 1 patient (2%) had a history of heart disease, and 1 patient (2%) had diabetes.
4.4. Clinical Symptoms
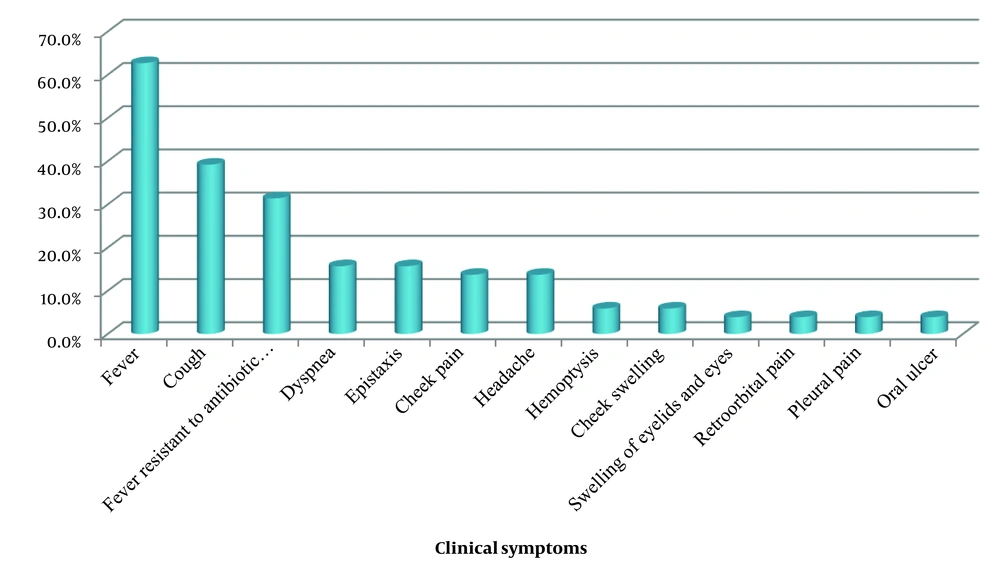
Among the 51 patients with IA and HM, the most common symptoms were fever (n = 23, 62.7%), cough (n = 20, 39.2%), and fever resistant to antibiotic therapy (n = 16, 31.4%) (Figure 1).
4.5. Radiological Findings
All patients exhibited abnormal findings on chest CT scans. The predominant finding was nodules with a halo sign (n = 31, 72.1%), followed by nodules without a halo sign (n = 12, 27.9%), cavities (n = 8, 18.6%), wedge-shaped lesions (n = 7, 16.3%), and the air crescent sign (n = 4, 9.3%). Additionally, 45 patients (88.2%) underwent sinus CT scans, of whom, 35 patients (77.8%) had abnormal findings. Specifically, 3 patients (8.6%) showed evidence of orbital invasion, and 7 patients (20%) displayed evidence of nasal septum destruction. According to these results, 34 patients (66.7%) were diagnosed with sinopulmonary involvement, 14 patients (27.5%) with pulmonary involvement, and 3 patients (5.9%) with sinus-orbital involvement.
4.6. Treatment and Outcomes
At the initiation of antifungal medication for IA treatment, 27 patients had neutropenia (absolute neutrophil count < 500/mm3). All 51 patients had a history of receiving antibiotics before commencing antifungal treatment. Initially, liposomal amphotericin B or caspofungin was administered as empiric antifungal therapy. Subsequently, treatment was switched to voriconazole once the diagnosis of aspergillosis was considered probable or proven. Among the 51 patients with HM and IA, 8 patients succumbed to the illness, resulting in a mortality rate of 15.6%. AML (n = 5, 62.5%) was the most common hematologic malignancy associated with mortality. Demographic indices, clinical symptoms, radiological features, and underlying diseases did not exhibit an increased risk of mortality (P > 0.05).
5. Discussion
We conducted this study to provide insights into patients with IA and HM. We found that 14.6% of patients with HM were diagnosed with IA. In a similar study conducted in Shiraz, southern Iran, the incidence of IA diagnosed by PCR-enzyme-linked immunosorbent assay (ELISA) in patients with HM was 7.2%. This difference in incidence could be attributed to the lower sensitivity of the PCR-ELISA test (12).
The incidence of IA in patients with HM varies among different medical centers, ranging from 8.9% to 22.14% (1, 4). This variation may be attributed to factors such as geographical location, the method of detection, and particularly the choice of antifungal prophylaxis agents. In our study, all patients received fluconazole as antifungal prophylaxis. Similarly, in a report on HM patients who underwent transplantation, with a fungal infection rate of about 10%, fluconazole was administered as the antifungal prophylaxis (13). However, two large studies have demonstrated significant reductions in the incidence of IFIs and IFI-related mortality when using posaconazole as a prophylactic treatment, compared to other azole drugs, in patients undergoing HSCT or in those with AML and MDS (14, 15).
Consistent with findings from previous reports in Iran (16) and Australia (17), AML was the most common malignancy observed in HM patients with IA in our study. Additionally, our study revealed that IA was more frequent in males than in females, a trend observed in some previous studies (18-20). This gender difference may be explained by the epidemiological characteristics of underlying diseases, as IA is more prevalent in certain conditions. For instance, HM, which is strongly associated with IA, is more common in men than in women (10). Regarding hospitalization, our study showed a mean length of stay of 104.9 days, which was longer compared to other studies, where this period ranged from 26.9 to 36 days (21, 22). Further analysis of the patient's records revealed that the extended hospitalization period was primarily due to the management of HM.
In the current investigation, the most common symptoms were fever (62.7%), cough (39.2%), and fever resistant to antibiotic therapy (31.4%). An earlier report on the clinical symptoms of IA also identified fever unresponsive to antibiotics as the most common clinical symptom of this fungal infection (23). Although various studies have reported A. fumigatus as the primary cause of IA (2), we identified A. flavus as the predominant etiological agent of IA, which is consistent with other reports from Iran (3, 24). Some studies have also reported A. tereus as an emerging agent for IA, mainly isolated from the lungs (25, 26). However, in our study, no A. tereus was isolated, while A. niger was found in three patients. Similar studies have also isolated A. niger in a small percentage of HM patients (27, 28). The results of a study by Alsalman et al. showed that the lungs were the most common site of IA involvement (29), whereas in the present study, sinopulmonary involvement was predominant. Clinical diagnosis of IA in HM patients is challenging due to the wide range of clinical manifestations (30).
The mortality rate of 15.6% reported in this study is lower than the mortality rates of 34% and 61% reported by Perkhofer et al. (17) and Husain et al. (31), respectively. The main factors influencing the outcome of patients with IA are underlying conditions, predisposing factors, and disease progression in the affected organs. Previous studies have shown that liver or bone marrow transplantation, diabetes, and neutropenia are associated with an increased risk of mortality. In our study, AML was the most common hematologic malignancy among patients who died.
The main limitation of this study was the inability to perform invasive diagnostic procedures to obtain tissue samples due to coagulation disorders in many patients. Additionally, the absence of antifungal susceptibility testing and the retrospective nature of the study were other limitations. Larger prospective studies with precise fungal identification and antifungal susceptibility testing are essential in this field.
5.1. Conclusions
Our findings showed that the incidence of IA in HM patients was relatively high, showing the lack of anti-mold prophylaxis in these patients. Anti-mold prophylaxis is particularly recommended in patients with AML, in whom the IA incidence and mortality rate was reported to be higher. A. flavus was the most common isolated species, which shows a different epidemiological pattern of Aspergillosis species in Iranian patients.