1. Background
Since the declaration of COVID-19 as a pandemic by the World Health Organization in March 2020, it has become the highest healthcare priority globally. This disease has impacted over 255 million people worldwide and resulted in the deaths of more than 5 million patients globally (1). Individuals displaying extrapulmonary symptoms of COVID-19 can significantly contribute to community transmission of the disease (2). The primary diagnostic tool for COVID-19 currently relies on real-time polymerase chain reaction (RT-PCR) testing of SARS-CoV-2 using nasopharyngeal and oropharyngeal samples in conjunction with lung CT scans (3). However, previous studies have suggested repeated RT-PCR testing on nasopharyngeal samples to enhance diagnostic accuracy (4). Given the emergence of new virus variants amid widespread vaccination efforts, identifying the most effective diagnostic tool for this disease remains crucial in the fight against the pandemic (5).
Prior literature has shown that RT-PCR conducted on anal or rectal swabs can yield positive results in COVID-19 patients even after the conversion of their naso- and oropharyngeal swabs, indicating the potential for fecal-oral transmission when pharyngeal PCR tests are negative (6, 7). Additionally, higher viral loads in anal swabs have been linked to worse outcomes (8). The use of RT-PCR on saliva samples has also been explored for COVID-19 detection, although its diagnostic value is still being investigated. This method of specimen collection is much more convenient than conventional pharyngeal swab sampling, potentially improving patient compliance with specimen collection (9).
2. Objectives
This study aimed to compare the effectiveness of single and double RT-PCR testing on nasopharyngeal and oropharyngeal swabs, as well as anal swabs and saliva samples, for the diagnosis of COVID-19.
3. Methods
3.1. Study Design and Participants
This study followed a cross-sectional protocol involving 102 pediatric patients referred to the pediatric infectious diseases clinics at Shiraz University of Medical Sciences (SUMS). Inclusion criteria encompassed all symptomatic patients referred to SUMS pediatric infectious diseases clinics suspected of COVID-19, while exclusion criteria included patients or their parents expressing unwillingness to participate in the study. After the routine collection of oropharyngeal and nasopharyngeal swabs for detecting SARS-CoV-2 infection, patients were requested to undergo another round of specimen collection from the oropharyngeal and nasopharyngeal areas. Additionally, an anal swab was obtained, and saliva specimens were collected from the patients.
3.2. Real-time Polymerase Chain Reaction
Viral genome extraction was performed using the SinaPure Viral Kit (SinaClon BioScience, Iran), following the kit's instructions. The SARS-CoV-2 Test Kit utilized an in vitro molecular diagnostic method employing Taq Man's probe-based technology for detecting SARS-CoV-2. To minimize the risk of sample contamination during the assay run, a negative (no template) control was included. Similarly, a positive template control was used to confirm that the assay run proceeded as planned on each tested plate.
3.3. Statistical Analysis
The obtained results were entered into IBM SPSS 27. Quantitative variables were presented as the mean and standard deviation, while qualitative variables were reported as frequency and percentage. A positive RT-PCR result from nasopharyngeal and/or oropharyngeal swabs served as the reference standard (gold standard). Other specimen collection methods, including single collections from the nasopharynx and oropharynx, dual collections from the nasopharynx and oropharynx, saliva, and anus, were evaluated in comparison to the gold standard. For each test, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated, and agreement was measured using the Kappa statistic for each comparison. Statistical significance was determined with a threshold of P-values less than 0.05.
4. Results
A total of 102 suspected COVID-19 cases were included in the study, comprising 48 (47.1%) males and 54 (52.9%) females. The mean age of the patients was 31.42 ± 28.378 months. Pharyngeal RT-PCR tests produced positive results in 83 patients (81.4%) and negative results in 19 patients (18.6%). In a subsequent round of testing, 86 positive results were obtained, with 75 of them being consistent with the first round. Table 1 summarizes the demographic information of the population and the prevalence of COVID-19 based on different diagnostic methods.
| Variables | Values |
|---|---|
| Sex | |
| Male | 48 (47.1) |
| Female | 54 (52.9) |
| Age, mo | 31.42 ± 28.378 |
| Prevalence of COVID-19 | |
| Single nasopharyngeal swab | 83 (81.4) |
| Double nasopharyngeal swab | 94 (92.2) |
| Anal swab | 91 (89.2) |
| Saliva | 92 (90.2) |
a Values are expressed as mean ± SD or No. (%).
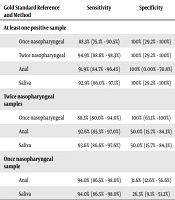
When considering twice-pharyngeal testing as the gold standard, the sensitivity, specificity, PPV, and negative predictive value for a single pharyngeal sample was 88.3% (95% CI: 80.0% - 94.0%), 100% (95% CI: 63.1% - 100%), 100% (95% CI: 95.7% - 100%), and 42.1% (95% CI: 20.3% - 66.5%), respectively, in a population with a prevalence of 92.2%. The Kappa measure of agreement was 0.542 (P < 0.001), indicating moderate agreement.
In the RT-PCR of anal swabs, 91 patients (89.2%) tested positive. When a single pharyngeal sample was considered the gold standard, the sensitivity, specificity, PPV, and NPV for anal swabs were 94.0% (95% CI: 86.5% - 98.0%), 31.6% (95% CI: 12.6% - 56.6%), 85.7% (95% CI: 76.8% - 92.2%), and 54.5% (95% CI: 23.4% - 83.3%), respectively. The Kappa measure of agreement was 0.122 (P = 0.001), signifying a significant but weak agreement.
When two samples from the nasopharynx and/or oropharynx were set as the gold standard, the sensitivity, specificity, PPV, and NPV of anal swabs changed to 92.6% (95% CI: 85.3% - 97.0%), 50.0% (95% CI: 15.7% - 84.3%), 95.6% (95% CI: 89.1% - 98.8%), and 36.4% (95% CI: 10.9% - 69.2%), respectively. The Kappa measure of agreement was 0.151 (P < 0.001), statistically significant but clinically irrelevant. Saliva RT-PCR yielded positive results in 92 patients, with 78 having previously tested positive pharyngeally. Considering the first pharyngeal test as the gold standard, the sensitivity, specificity, PPV, and NPV of saliva RT-PCR were 94.0% (95% CI: 86.5% - 98.0%), 26.3% (95% CI: 9.1% - 51.2%), 84.8% (95% CI: 75.8% - 91.4%), and 50.0% (95% CI: 18.7% - 81.3%), respectively. The Kappa measure of agreement was 0.121 (P = 0.007), statistically significant but clinically irrelevant.
Changing the gold standard to two pharyngeal samples, the sensitivity, specificity, PPV, and NPV of saliva RT-PCR altered to 93.6% (95% CI: 86.6% - 97.6%), 50.0% (95% CI: 15.7% - 84.3%), 95.7% (95% CI: 89.2% - 98.8%), and 40.0% (95% CI: 12.2% - 73.8%), respectively. The Kappa measure of agreement for these tests was 0.155 (P < 0.001), clinically irrelevant but statistically significant. Table 2 summarizes the comparison of different diagnostic methods in this study.
| Gold Standard Reference and Method | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Kappa | P-Value |
|---|---|---|---|---|---|---|
| At least one positive sample | ||||||
| Once nasopharyngeal | 88.3% (75.1% - 90.5%) | 100% (29.2% - 100%) | 100% (95.7% - 100%) | 21.45% (3.4% - 39.6%) | 0.234 | < 0.001 |
| Twice nasopharyngeal | 94.9% (88.6% - 98.3%) | 100% (29.2% - 100%) | 100% (96.2% - 100%) | 37.5% (8.5% - 75.5%) | 0.525 | < 0.001 |
| Anal | 91.9% (84.7% - 96.4%) | 100% (0.00% - 70.8%) | 100% (96.0% - 100%) | 27.3% (6.0% - 61.0%) | 0.163 | < 0.001 |
| Saliva | 92.9% (86.0% - 97.1%) | 100% (29.2% - 100%) | 100% (96.1% - 100%) | 30.0% (6.7% - 65.2%) | 0.436 | < 0.001 |
| Twice nasopharyngeal samples | ||||||
| Once nasopharyngeal | 88.3% (80.0% - 94.0%) | 100% (63.1% - 100%) | 100% (95.7% - 100%) | 42.1% (20.3% - 66.5%) | 0.542 | < 0.001 |
| Anal | 92.6% (85.3% - 97.0%) | 50.0% (15.7% - 84.3%) | 95.6% (89.1% - 98.8%) | 36.4% (10.9% - 69.2%) | 0.151 | < 0.001 |
| Saliva | 93.6% (86.6% - 97.6%) | 50.0% (15.7% - 84.3%) | 95.7% (89.2% - 98.8%) | 40.0% (12.2% - 73.8%) | 0.155 | < 0.001 |
| Once nasopharyngeal sample | ||||||
| Anal | 94.0% (86.5% - 98.0%) | 31.6% (12.6% - 56.6%) | 85.7% (76.8% - 92.2%) | 54.5% (23.4% - 83.3%) | 0.122 | 0.001 |
| Saliva | 94.0% (86.5% - 98.0%) | 26.3% (9.1% - 51.2%) | 84.8% (75.8% - 91.4%) | 50.0% (18.7% - 81.3%) | 0.121 | 0.007 |
5. Discussion
In this study, 102 patients suspected of COVID-19 who were referred to the pediatric infectious diseases clinics at Shiraz University of Medical Sciences were included. The patients underwent PCR testing on samples from their pharynx, anus, and saliva. While testing nasopharyngeal and/or oropharyngeal samples, double specimen collection proved more effective in diagnosing COVID-19, but it was not deemed cost-effective. Results from PCR on anal swabs and saliva were not clinically relevant when compared to pharyngeal swabs. Despite some patients having positive PCR tests in their anal swabs and saliva with two negative pharyngeal tests, the use of anal swabs and saliva tests was considered not cost-effective during the pandemic. However, during epidemic resolution, when case finding is crucial, these methods might be employed for more thorough investigations.
This study focused specifically on pediatric patients due to the inherent challenges associated with proper specimen collection from the nasopharynx or oropharynx in this demographic. Collecting specimens from pediatric patients poses greater difficulty compared to the adult population, potentially impacting the clinical judgment of physicians and posing a higher risk of disease transmission within the community (10). In a study conducted by Li et al., RT-PCR for SARS-CoV-2 in anal swabs was found to be positive in approximately one-fifth of patients with COVID-19 at the time of admission. During hospitalization, this percentage increased to about 30%. This elevated positivity rate suggests the potential occurrence of viral shedding from the gastrointestinal tract. Notably, the positive results from anal swab RT-PCR for SARS-CoV-2 were associated with negative anti-nucleocapsid serology. This association could be attributed to the fact that individuals with decreased immunity and weaker immune systems may exhibit lower antibody titers. Consequently, patients with such conditions might harbor higher viral loads in their gastrointestinal tract, potentially serving as more potent sources for the spread of infection (10).
In a study conducted by Abdullah et al. on adult Indonesian patients, the sensitivity of anal swab RT-PCR was reported to be only 36.7% compared to pharyngeal swabs, despite exhibiting high specificity. This observed difference contrasts with our study, as pediatric patients typically possess weaker immune systems than their adult counterparts. Consequently, pediatric patients may manifest higher viral shedding from their feces and gastrointestinal tract, potentially influencing the sensitivity of anal swab RT-PCR in this specific population (11).
Qiu et al. discovered that anal swabs and saliva specimens could serve as criteria for hospital discharge due to the prolonged time required for seroconversion compared to oropharyngeal and nasopharyngeal swabs. However, given the high incidence of false-negative results in anal swabs and saliva samples, they recommended the simultaneous use of all sampling methods for a more comprehensive and reliable assessment (12).
In the study conducted by Li et al., a correlation was observed between higher viral loads in anal swabs, as opposed to viral loads in pharyngeal swabs, and increased mortality rates. Consequently, anal swabs may serve as prognostic factors in COVID-19 patients. Particularly in adult patients, the presence of positive anal swab specimens might indicate a more systemic disease. Elevated viral loads are typically observed in the initial and later stages of COVID-19, given that the respiratory system serves as the primary replication site of SARS-CoV-2 (8). Pasomsub et al. discovered that saliva samples exhibited proper sensitivity and specificity for detecting SARS-CoV-2 infection in a cohort of 200 Thai patients. Their study reported an agreement rate of 97.5% between pharyngeal swabs and saliva samples. These findings align with the outcomes observed in our study (13).
In a study conducted by Zhu et al. on a large cohort of patients in China, it was revealed that saliva RT-PCR demonstrated diagnostic value comparable to that of pharyngeal swabs. However, the prognostic value of this method was questioned (14). According to a review article by Fernandes et al., salivary samples were identified as suitable diagnostic tools for COVID-19, particularly when a noninvasive and cost-effective method is needed. This sampling approach could potentially serve as a screening tool in mass populations during the peak of COVID-19 and in times of pandemic resolution (9). It would have been preferable to conduct these tests in a more broadly representative population. Unfortunately, due to limitations in financial resources and equipment, achieving such generalizability was not feasible in this study. Future research efforts are encouraged to focus on samples that better mirror the prevalence of COVID-19 and the demographic characteristics of the general population. Additionally, further studies comparing RT-PCR results with quantitative viral loads of patients could provide additional insights into this matter.
5.1. Conclusions
Double sampling of nasopharyngeal and oropharyngeal swabs, along with anal swabs and saliva sampling, has proven effective in diagnosing COVID-19. However, the routine implementation of these tests may not be deemed cost-effective. Nevertheless, during periods of pandemic resolution when comprehensive case finding is crucial, these tests can be employed to more effectively mitigate and eliminate the disease from society.