1. Introduction
Vibrio vulnificus is a species of rare pathogenic marine bacteria associated with necrotizing fasciitis, septic shock, and other severe diseases. If the infection is not treated in time, then the risks of amputation and the fatality rate are high due to its acute onset and rapid deterioration. However, given that sporadic V. vulnificus infection is rare, most clinicians lack relevant experience in the diagnosis and treatment of this disease (1). This study introduced an antibacterial treatment for a patient with V. vulnificus sepsis. The anti-infective treatment plans were formulated according to the infection site of the patient, pharmacokinetics/pharmacodynamics (PK/PD) characteristics of bacteria, and antibacterial drugs so that the patient could receive an effective treatment. This study aimed to accumulate experience regarding the clinical treatment of V. vulnificus infection and provide a reference for the formulation of a medication plan.
2. Case Presentation
A 73-year-old female patient was admitted to Wuxi People's Hospital on July 24, 2022. She visited the emergency department due to a sudden delayed response without obvious inducement. Laboratory test results were as follows: blood routine examinations were white blood cell (WBC) of 3.63 × 109/L, red blood cell (RBC) of 2.81 × 1012/L, lymphocyte (L) of 0.72 × 109/L, platelet count (PLT) of 97 × 109/L, neutrophil ratio (N%) 73.5%, and C-reactive protein (CRP) < 0.5 mg/L. The laboratory results are summarized in Table 1. The patient had a history of penicillin allergy. Upon admission, the patient had a temperature (T) of 37.1°C, a heart rate (HR) of 78 times/min, a respiration rate (R) of 16 times/min, and a blood pressure (BP) of 150/80 mmHg. The patient was diagnosed with cerebral infarction and was admitted to the Department of Neurology for further treatment. Magnetic resonance imaging (MRI) examination showed multiple cerebral infarctions in the right frontal lobe.
| Hospital Day | 1 | 2 | 8 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 22 | 25 | 26 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory Data | ||||||||||||||||
| WBC count (× 109/L) | 3.63 | 3.21 | 2.96 | 6.38 | 2.43 | 5.43 | 7.4 | 6.9 | 4.12 | 3.13 | 2.94 | 3.36 | 3.44 | 3.82 | 4.23 | 4.54 |
| Hemoglobin (g/L) | 102 | 106 | 89 | 89 | 78 | 89 | 81 | 104 | 101 | 88 | 90 | 90 | 92 | 86 | 69 | 73 |
| PLT (× 109/L) | 97 | 107 | 73 | 62 | 36 | 21 | 34 | 24 | 48 | 56 | 46 | 49 | 49 | 82 | 95 | 100 |
| RBC count (× 1012/L) | 2.81 | 2.92 | 2.43 | 2.44 | 2.13 | 2.59 | 2.34 | 3.13 | 2.05 | 2.55 | 2.65 | 2.57 | 2.61 | 2.48 | 1.99 | 2.09 |
| BUN (mmol/L) | 4.9 | - | - | - | 13.1 | 15.6 | 17.4 | 19.3 | 19.1 | 14.7 | 11 | - | - | 8.3 | 4.1 | 5.3 |
| Creatinine (umol/L) | 46 | - | - | - | 179.4 | 148.1 | 108.2 | 98.7 | 76 | 52.8 | 46.1 | - | - | 49.5 | 48.2 | 47.7 |
| Na (mmol/L) | 137.9 | - | - | - | 134 | 136.8 | 141.7 | 146.9 | 148.1 | 148.4 | 140.8 | - | - | 139.5 | 130.8 | 135.1 |
| K (mmol/L) | 4.03 | - | - | - | 3.27 | 2.97 | 3.27 | 3.85 | 4.62 | 4.05 | 4.38 | - | - | 4.78 | 3.68 | 4.34 |
| AST (U/L) | 23 | - | - | - | 64 | 73 | 25 | - | 34 | 35 | 28 | - | - | 27 | 24 | - |
| ALT (U/L) | 14.9 | - | - | - | 32.5 | 35.9 | 27.8 | - | 30.1 | 31.6 | 30.9 | - | - | 25.3 | 17.5 | - |
| CRP (mg/L) | - | - | - | 52.6 | 232.3 | 256 | 197.7 | 153 | 109.2 | 83.4 | 63.8 | 35.4 | 32.2 | 26.4 | 20.1 | - |
| PCT (ng/mL) | - | - | - | - | 150 | 90.91 | 40.88 | 28.38 | 7.02 | - | 1.06 | 0.76 | - | - | 0.11 | - |
| CK MB (Active) (U/L) | 12 | - | - | - | 17.5 | 49.5 | 7 | 7.1 | - | - | 3.4 | - | - | - | - | - |
| HT I (ug/L) | <0.012 | - | - | - | - | 10 | 2.74 | 1.18 | 0.633 | - | 0.193 | - | - | 0.166 | 0.091 | - |
| CK (U/L) | 29 | - | - | - | - | 795 | 340 | 314 | - | - | - | - | - | - | 68 | - |
| CK MB (Mass) (ng/mL) | 0.54 | - | - | - | - | 34.5 | 3.65 | 1.83 | 0.65 | - | 0.64 | - | - | 2.45 | 3.53 | - |
| D-dimer (ug/L) | 190 | 268 | - | - | 4630 | 1108 | 916 | - | 3118 | - | 3381 | - | - | 2464 | 818 | - |
| NT-proBNP (pg/mL) | - | - | - | - | - | 30546 | - | - | - | - | 9781 | 3199 | - | - | 291 | - |
Laboratory Examination Results in the Hospital
The patient complained of obvious pruritus all over the body with visible scratches on the 2nd day of admission. Blood routine results showed that WBC, RBC, L, and PLT were all lower than the normal range. The patient’s pruritus disappeared on the 6th day. The patient’s memory recovered on the 9th day. Chills and fever occurred at night, and the highest body temperature was recorded to be 38.9°C. The patient developed redness, swelling, heat, and pain in the right lower limb, which were accompanied by several episodes of nausea and vomiting. Her body temperature reached 39.4°C, and the local skin temperature rose with swelling of the right lower leg on the 10th day. Blood routine results showed that the counts of WBC, RBC, L, PLT, CRP, and creatine kinase (CK) were 6.38 × 109/L, 2.44 ×1012/L, 0.44 × 109/L, 62 × 109/L, 52.6 mg/L, and 964 U/L, respectively. Considering the infection of the right lower limb soft-tissue indicated by ultrasound, cefpiramide of 1 g bid combined with moxifloxacin of 0.4 g qd was given to prevent infection.
The patient was listless and had a fever with the highest temperature of 38.3°C on the 11th day. The swelling of the right lower limb did not subside, which was accompanied by aggravated pain. White blood cells, RBC, L, and PLT were all lower than the normal range; nevertheless, CRP and procalcitonin (PCT) were elevated. Heart, liver, kidney, and coagulation indexes were abnormal. Blood culture results showed the growth of gram-negative bacteria. Cefpiramide was discontinued; however, meropenem of 0.5 g q12h combined with moxifloxacin of 0.4 g qd was used for anti-infection. The patient suddenly became unconscious and did not respond to the call. The patient monitoring showed HR of 129 times/min, R of 30 times/min, and BP of 89/45 mmHg. She was immediately transferred to the intensive care unit (ICU) for treatment.
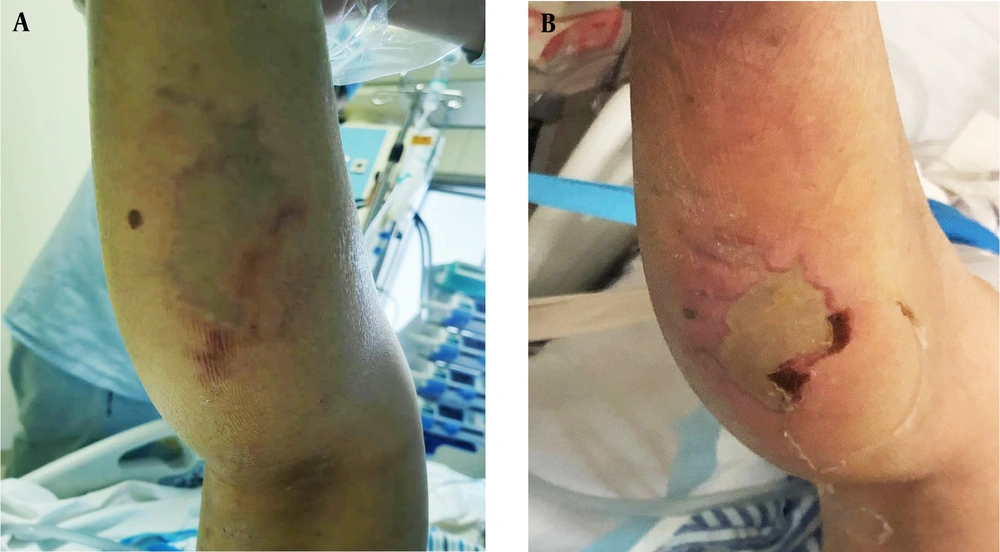
After admission to the ICU, emergency tracheal intubation was connected to a ventilator to assist ventilation. The right lower limb of the patient was partially red and pale in the center (Figure 1A). The ICU diagnosis results were cerebral infarction, right leg soft tissue infection, and septic shock. The patient had the highest temperature of 38.3°C, HR of 99 times/min, R of 18 times /min, and BP of 129/58 mmHg on the 12th day. The blood cultures showed that V. vulnificus was positive (Table 2) on the 13th day. Lymphocyte subpopulation count showed lymphocyte percentage of 4.07%, lymphocyte count of 306 cells/μL, CD3 total T cell count of 202 cells/μL, CD3+CD4+ helper T cell count of 116 cells/μL, and CD3+CD8+ cytotoxic T cell count of 76 cells/μL. Immunofunctional test showed immunoglobulin G (IgG) of 8.22 g/L, complement C3 of 566 mg/L, and interleukin-6 of 408.18 pg/mL. The patient had no history of eating raw seafood or contacting seawater before the onset of the disease, which was 1 week ago.
| Vibrio vulnificus +++ | ||
|---|---|---|
| Antibiotics | MIC (ug/mL) | Results |
| Imipenem | ≤ 1 | Sensitive |
| Ampicillin | ≤ 2 | Sensitive |
| Ampicillin/sulbactam | ≤ 2 | Sensitive |
| Cefazolin | 16 | Resistance |
| Ceftazidime | ≤ 1 | Sensitive |
| Cefepime | ≤ 1 | Sensitive |
| Cefotetan | 16 | Sensitive |
| Amikacin | 8 | Sensitive |
| Gentamicin | 2 | Sensitive |
| Ciprofloxacin | ≤ 0.25 | Sensitive |
| Levofloxacin | ≤ 0.25 | Sensitive |
| SMZco | ≤ 20 | Sensitive |
| Piperacillin/tazobactam | ≤ 4 | Sensitive |
Drug Sensitivity Test of Vibrio vulnificus
The pale area of skin in the center of the right calf was observed to be larger on the 16th day. The WBC, RBC, L, and PLT were all lower than normal; however, CRP and PCT were still higher than normal. The indicators of renal function were abnormal. The clinical pharmacists suggested a sufficient combination of quinolones based on third-generation cephalosporin to enhance the anti-infection treatment. Therefore, the dose of meropenem was adjusted to 1 g q8h and was combined with levofloxacin sodium chloride injection for anti-infection of 0.5 g qd. On the 18th day, the local swelling, heat, and pain of the right lower limb were reduced. Meropenem was stopped and replaced with cefoperazone sodium/sulbactam sodium of 3 g q8h combined with levofloxacin of 0.5 g qd for anti-infection.
The redness, swelling, heat, and pain in the right calf were significantly relieved on the 20th day (Figure 1B). On the 22nd day, the pale skin area of the patient’s right lower limb was reduced, with surrounding redness and swelling accompanied by slight tenderness. Ultrasound showed local swelling-like changes. Orthopedic surgeons performed local incision decompression of the ruptured wound at the back of the right lower limb and then discharged a large number of purulent secretions. The diagnosis of necrotizing fasciitis was confirmed intraoperatively. The patient’s right lower limb pain improved on the 25th day of admission. The bacterial culture of the wound became sterile. The pain in the right lower limb was no longer noticeable, and the pale area of the skin was further reduced on the 28th day. Therefore, the patient was discharged. The time sequence of key events is presented in Figure 2.
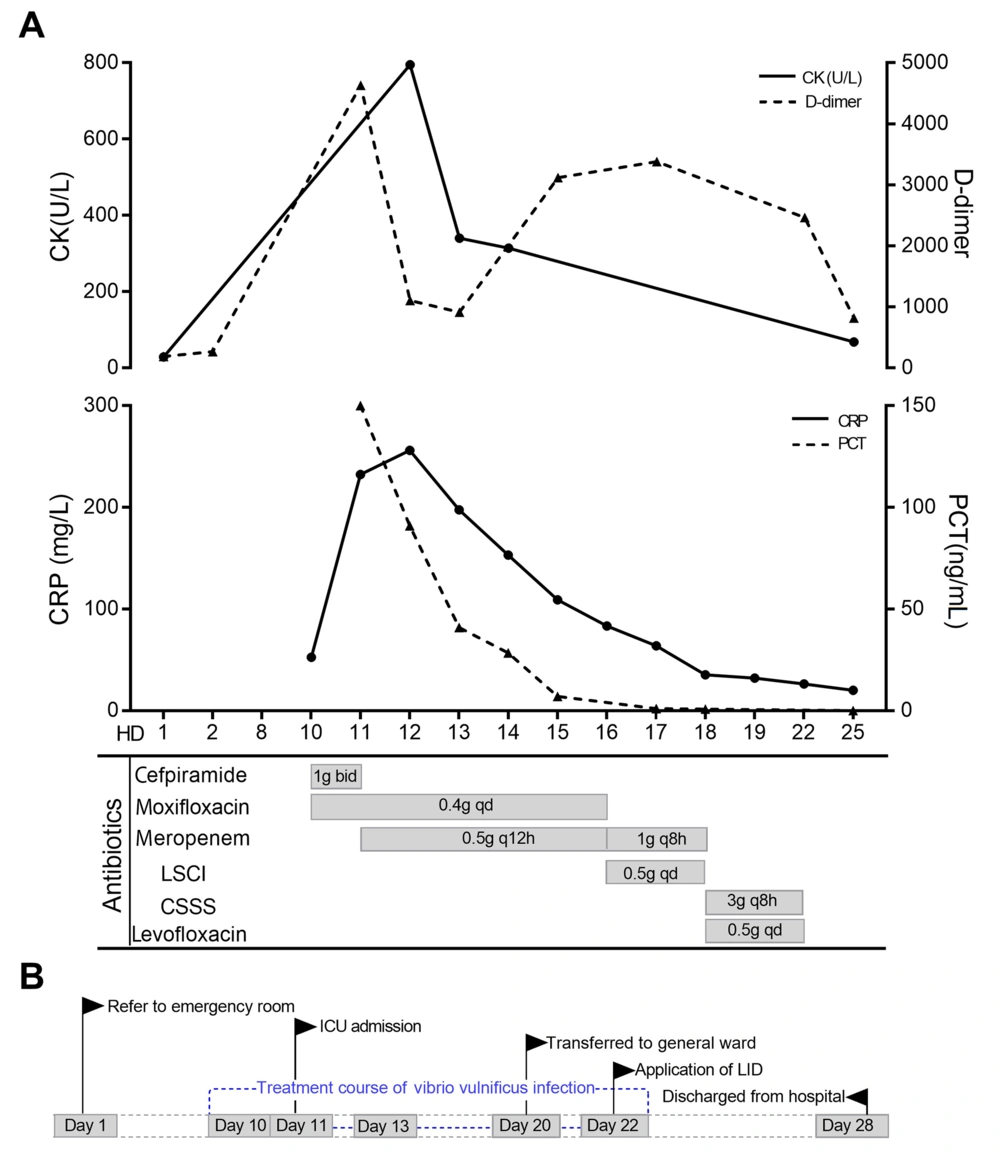
Time sequence of key events. A, clinical course of antibiotic use during V. vulnificus infection; B, key events of the patient during admission. Abbreviations: CK, creatine kinase; CRP, C-reactive protein; PCT, procalcitonin; HD, hospital day; LSCI, levofloxacin sodium chloride injection; CSSS, cefoperazone sodium/sulbactam sodium; ICU, intensive care unit; LID, local incision decompression.
3. Discussion
The incidence of V. vulnificus infection had obvious seasonality, which was more common in a warm climate and more in summer than in winter. The incidence of this case was within July to August, which is consistent with the seasonal characteristics of V. vulnificus infection. The population susceptible to V. vulnificus mainly had chronic liver/kidney disease, hemochromatosis, long-term alcoholism, and other immunocompromised functions (1, 2). The blood routine of the patient showed that the three-line cells decreased, and bone marrow aspiration indicated a high probability of myelodysplastic syndrome or chronic atypical aplastic anemia. The results of immune function suggested that the patient had a low immune function and risk factors for V. vulnificus infection. The clinical manifestations of V. vulnificus infection were rapidly progressing local lesions and worsening general conditions. Most patients would present maculopapular rash, urticaria, and other skin lesions.
Muscle damage occurred within 24 - 48 hours after the onset, which commonly included severe local pain, swelling, skin ecchymosis, blood blisters, and necrosis of lower limbs aggravated and expanded within hours. Most patients rapidly developed signs and symptoms of multiple organ failure, which was accompanied by hypotension or shock. Fever was the most common clinical symptom, and pain and edema mostly occurred in the lower limbs (3, 4).
The patient showed pruritus all over the body, rapid progression of lower extremity redness, heat and pain, high fever, low BP, and liver and kidney insufficiency. These clinical manifestations are consistent with those of V. vulnificus sepsis. Serum creatine kinase significantly increased after V. vulnificus infection, and the increased level reflected the severity of local lesions. Vibrio vulnificus could be isolated from blood and other cultures, and this approach was the gold standard for clinical diagnosis (1). Creatine kinase rapidly increased from 29 U/L to 1313 U/L, the D-dimer index increased from 190 ug /L to 4 630 ug /L, and the abnormal coagulation function index indicated the progression to disseminated intravascular coagulation at the onset of the disease. The results of blood cultures were reported as V. vulnificus, which was consistent with the laboratory manifestations of V. vulnificus infection.
Vibrio vulnificus has flagella, pili and capsule. Water-soluble antibiotics could not passively diffuse into the plasma membrane of eukaryotic cells, which results in weaker intracellular bactericidal action than that of lipid-soluble drugs. Therefore, lipid-soluble drugs with good tissue permeability and relatively high concentration should be selected for skin and soft tissue infection (4). Ceftriaxone/cefotaxime combined with doxycycline was recommended for V. vulnificus treatment by Stevens et al. (5). Trinh et al. showed that cephalosporins combined with quinolones had the best therapeutic effect for V. vulnificus infection (6). Meropenem was selected for anti-infection treatment based on the results of drug sensitivity and considering septic shock and severe infection. The patient’s body temperature decreased, CRP decreased from 197.7 mg/L to 83.4 mg/L, and PCT decreased from 40.8 ng/mL to 7.02 ng/mL on the 16th day (Figure 2A). However, the local redness and swelling of the patient’s right lower limb showed a tendency to spread and aggravate, and the pale area of the skin was larger than before.
Meropenem had poor tissue permeability due to its lower ratio of area under the curve (AUC) in skin and soft tissue to AUC in plasma than that of lipophilic drugs, which might be part of the reason for the decrease in infection index and the aggravation of local redness. Fluoroquinolones of strong lipophilicity and good tissue permeability could be used for skin and soft tissue infection (7). It was considered to be combined with quinolones based on the third generation of cephalosporin for anti-infection. Therefore, cefoperazone sodium and sulbactam sodium combined with levofloxacin were used for treatment. In the end, the reswelling and heat pain of the right lower limb were significantly relieved. Therefore, adverse clinical outcomes, such as amputation, were avoided. Antibiotics for uncomplicated bloodstream infections should generally be administered until 7 - 10 days after body temperature returns to normal (8). The patient took the drug until 9 days after the body temperature returned to normal, and this course of treatment was reasonable (Figure 2B).
3.1. Conclusions
The patient had no clear epidemiological history of V. vulnificus infection and no vibrio infection-related prodrome symptoms at admission. Considering the patient’s clinical manifestations and etiological evidence, she was diagnosed to have a V. vulnificus infection. After the anti-infection treatment with meropenem, the patient’s infection indexes gradually decreased; however, the lower extremity lesions tended to aggravate. Therefore, the clinical pharmacists suggested changing to third-generation cephalosporins combined with fluoroquinolones for anti-infection. After 5 days of treatment, the patient’s lower extremity lesions improved. When the ultrasound of the lower extremities showed indications for surgical intervention, local incision decompression and drainage were performed in time. The patient's infection was managed, and the local lesions were further reduced. This case suggests that, for V. vulnificus infection, we not only need to refer to the drug susceptibility results but also need to formulate anti-infection treatment plans based on various factors, such as the patient’s infection site and PK/PD characteristics. Meanwhile, surgical debridement intervention is also important in patients with V. vulnificus infection.