1. Background
In December 2019, a new lung-related disease was reported in Wuhan, China, by Health Organizations, and it was officially named Coronavirus disease 2019 (COVID-19) (1). During the COVID-19 pandemic, healthcare workers (HCWs) were at the frontline of care for patients suspected or officially diagnosed with COVID-19 despite insufficient training or personal protective equipment (PPE). The risk among HCWs was high during the initial months of the pandemic (2-4). The pandemic is characterized by the rapid development of vaccines using conventional and new platforms, with immediate use to immunize the world population (5). Although different vaccines can significantly reduce hospitalization and mortality, breakthrough infections have been reported with all the vaccines used (6-8). The symptoms of the disease in breakthrough-infected individuals or those who have been re-infected can vary from mild to severe (9, 10).
SARS-CoV-2 is an enveloped virus with a positive-sense, single-stranded (SS), and non-segmented RNA genome that exhibits an ever-evolving nature, resulting in the emergence of multiple viral variants with alterations in genomic or amino acid profiles. Among the most important variants are those occurring in the spike protein region of the virus, which can lead to evasion of the host immune system or increased binding affinity of the receptor-binding domain (RBD) of the virus to its host angiotensin-converting enzyme II (ACE2) receptors. However, due to ongoing changes in the form of single nucleotide polymorphisms (SNPs) and viral mutations, controlling and predicting its spread is crucial. Some mutations directly affect the pathogenicity of COVID-19 and the transmission rate (11).
Important mutations in the RBD of the SARS-CoV-2 virus can lead to changes in the pathogenicity of the virus and disease severity. The formation of new variants of the SARS-CoV-2 genome, which contains the S protein necessary for ACE2 receptor binding and fusion, is crucial. The large structure S glycoprotein of SARS-CoV-2 consists of two subunits: A receptor-binding fragment (S1) and a fusion fragment (S2). S1 consists of three domains: N-terminal domain (NTD), ACE2 receptor domain, and C-terminal domain (CTD) (12-14).
In a study conducted in Iran, researchers investigated variations occurring in the spike protein of SARS-CoV-2, particularly in the receptor-binding domain (RBD). By analyzing samples from Iranian patients, they identified mutations like A475V, L452R, V483A, and F490L within this region. These mutations potentially alter the virus's functionality and its interaction with antibodies (15). Additionally, Eslami et al. investigated 176 high-quality S glycoprotein sequences of Iranian SARS-CoV-2 from public databases between April 2020 and May 2021. They identified various mutations and discussed their potential impact on infectivity and response to neutralizing antibodies. Notably, the Delta variant showed higher transmissibility in Iran and was predicted to become dominant, with some instances carrying an additional mutation (E1202Q) potentially aiding viral-cell fusion. Common mutations like N-terminal domain (NTD) deletion at position I210 and P863H in the fusion peptide-heptad repeat 1 span region were also observed in Iranian SARS-CoV-2. These mutations hold significance in predicting disease spread and influencing vaccine and drug design to combat the virus (16).
The study of mutations occurring in the S protein is of great importance for conducting scientific research on therapeutic strategies considering the immune status induced by the vaccine and the occurrence of clinical symptoms with different severity. By analyzing mutations in critical regions of the S gene of the virus, we can examine the level of genetic diversity within this group of viruses and investigate whether vaccination has been able to control genetic variations. Furthermore, by examining the extent of mutations in different regions of the S gene, we can assess whether these mutations have any impact on disease severity and the occurrence of serious complications.
2. Objectives
This study was conducted to investigate the levels of mutations in the RBD, CTD, and NTD regions of the S gene of the SARS-CoV-2 virus in breakthrough-infected healthcare workers (HCWs) during the Omicron wave in Iran. Additionally, we compared the severity of clinical symptoms with mutations occurring at key sites of the S virus gene and monitored the antibody response, which can provide insights into infection rates among HCWs.
3. Methods
3.1. Study Design
This is an observational cross-sectional study conducted from November 2021 to May 2022. The study population consisted of healthcare workers (HCWs) from Shiraz University of Medical Sciences (SUMS) who had received the COVID-19 vaccination but were reinfected. The inclusion criteria for this study were HCWs aged 18 - 60 who had received a minimum of 2 doses of the COVID-19 vaccine and tested positive for SARS-CoV-2 using the rt-qPCR assay. A total of 49 HCWs were included in the study, and the sampling was done using the census method. Written informed consent was obtained from all study participants or their legally authorized representatives.
3.2. Sample Collection
Nasal and nasopharyngeal swabs were collected from the HCWs, and RNA extraction was performed using the ROJE extraction kit (ROJE, Iran) following the manufacturer's instructions. The presence of the virus in their throat and nasal samples was tested using real-time reverse transcription-polymerase chain reaction (rt-qPCR). Positive samples were stored in a freezer at -70°C until testing. A 5 mL blood sample was taken from the HCWs to assess their IgG antibody levels. Genetic evaluation of different regions of the S gene, including the RBD, CTD, and NTD regions, was conducted using the Nested PCR method. The primers used for different regions of the S gene are listed in Table 1.
| S Gene Segment and Oligo-Nucleotide | Sequences | Amplicon Size BP | Location of Primer | Tm |
|---|---|---|---|---|
| N-terminal domain | ||||
| SB5 Primary F | 5'-AACCAGAACTCAATTACCCCC | 21619-21639 | 59º | |
| SB6 Primary R | 5'-TTTGAAATTACCCTGTTTTCC | 505 | 22103-22123 | 54º |
| SB7 Nested F | 5'-TCAATTACCCCCTGCATACAC | 21628-21648 | 59º | |
| SB8 Nested R | 5'-ATTACCCTGTTTTCCTTCAAG | 490 | 22097-22117 | 55º |
| ACE2 receptor | ||||
| SS1 Primary F | 5'-TGTGTTGCTGATTATTCTGTC | 22643-22663 | 55º | |
| Binding domain | ||||
| SS2 Primary R | 5'-AAAGTACTACTACTCTGTATG | 460 | 23082-23102 | 54º |
| SS3 Nested F | 5'-ATTCTGTCCTATATAATTCCG | 22656-22676 | 54º | |
| SS4 Nested R | 5'-TACTCTGTATGGTTGGTAACC | 437 | 23072-23092 | 57º |
| C-terminal domain | ||||
| VF1 Primary F | 5'-AATCATTACTACAGACAACAC | 24901-24921 | 54º | |
| VF2 Primary R | 5'-CAATCAAGCCAGCTATAAAAC | 338 | 25218-25238 | 55º |
| VF3 Nested F | 5'-AGACAACACATTTGTGTCTGG | 24913-24933 | 57º | |
| VF4 Nested R | 5'-GCTATAAAACCTAGCCAAATG | 315 | 25207-25227 | 55º |
3.3. Genomic Analysis
We conducted a genomic analysis of the re-infected healthcare workers (HCWs) referred to as breakthrough-infected individuals. Initially, positive samples were screened using the SARS-CoV-2 nucleic acid kit. Subsequently, the positive samples' RNA was converted into cDNA using the Viragen kit. For cDNA synthesis, 20 µL reaction tubes were prepared. After thorough vortexing and spinning, the reaction tubes underwent thermal cycling in the thermocycler: First, a 10-minute incubation at 25°C, followed by a 60-minute incubation at 47°C, and finally, a 5-minute incubation at 85°C to conclude the RNA-to-cDNA synthesis. After completion, 1 µL of the synthesized cDNA was utilized for PCR amplification. The nested PCR method was used for evaluation, and gradient PCR was performed to determine the most suitable annealing temperature based on the type of primers used in each PCR reaction. Subsequently, 10 microliters of the second-round PCR products were electrophoresed on a 2% agarose gel, and the PCR products were sent to the virology laboratory at SUMS for sequencing to validate the study results.
3.4. Enzyme-Linked Immunoassay
An enzyme-linked immunoassay (ELISA) assay was conducted to measure anti-SARS-CoV-2 IgG levels in the serum of breakthrough-infected patients. The ELISA test employed the Pishtaz Teb kit, manufactured in Iran, to detect antibodies against the SARS-CoV-2 virus in the samples.
3.5. Mutational Analysis
Amplified fragments of the CTD gene were sent to the virology laboratory at REACTED for sequence determination. Sanger sequencing was employed to determine the fragment using the ABI3500 genetic analyzer. The obtained sequences were analyzed using Blast, and their accuracy was evaluated on NCBI. Validated sequences were then registered in the gene bank. BioEdit software was utilized for sequence analysis. Initially, low-quality nucleotides at the beginning and end of chromatograms were removed, and the entire sequences were corrected. Subsequently, both sequences were assembled, and any mismatches were identified. Finally, the corrected sequences were saved as FASTA files.
3.6. Clinical Presentation
Demographic characteristics, initial symptoms, clinical signs, and 60-day mortality rates were recorded and followed by a general physician.
3.7. Statistical Analysis
The collected data were entered into the statistical software SPSS version 21 and analyzed using the analysis of variance (ANOVA) method. In this study, a significance level of a P-value less than 0.05 was considered statistically significant.
4. Results
Based on the collected information, the nursing group comprised 27 individuals, representing 55.1% of the sample size. The medical group consisted of 6 individuals, accounting for 12.2% of the sample, and the service group comprised 16 individuals, representing 32.7% of the sample.
Additional information is presented in Table 2.
| Variables | No. (%) |
|---|---|
| Age, y | |
| Less than 25 | 8 (16.3) |
| 26 - 35 | 18 (36.7) |
| Above 36 | 23 (46.9) |
| Sex | |
| Male | 12 (24.5) |
| Female | 37 (75.5) |
| Type of vaccine | |
| Sinopharm | 17 (34.7) |
| AstraZeneca | 19 (38.8) |
| Sputnik V | 9 (18.4) |
| Bharat | 2 (4.1) |
| PastoCovac | 2 (4.1) |
| Result of antibody test | |
| Below 120 | 8 (16.3) |
| 121 - 170 | 10 (20.4) |
| 171 - 220 | 30 (61.2) |
| Above 221 | 1 (2) |
The results presented in Table 2 indicate that among the age distribution groups, the highest percentage, 46.9% (23/49), was observed in individuals aged 36 years and above. Regarding gender, women constituted the majority, accounting for 75.5% (37/49) of the cases. In terms of vaccine type, AstraZeneca had the highest usage rate at 38.8% (19/49).
As shown in Table 2, 16% of the breakthrough-infected HCWs had low IgG levels (≤120 IU), while none of them had IgG levels below 50 IU. It is noteworthy that the majority of them had high IgG antibody levels (63%). The most prevalent symptoms among the breakthrough-infected subjects were loss of smell sense, which was observed in 69.4% (34/49) of cases, followed by sore throat in 65.3% (32/49), headache in 59.2% (29/49), and cough in 57.1% (28/49). Shortness of breath was reported in 30.6% (15/49) of the breakthrough-infected HCWs (Table 3). No deaths were observed within 60 days after SARS-CoV-2 re-infection.
| Variables | Negative | Positive | P-Value |
|---|---|---|---|
| Fever | 26 (53.1) | 23 (46.9) | ≥ 0.05 |
| Headache | 20 (40.8) | 29 (59.2) | ≥ 0.05 |
| Losing smell sense | 15 (30.6) | 34 (69.4) | ≥ 0.05 |
| Sore throat | 17 (34.7) | 32 (65.3) | ≥ 0.05 |
| Shortness of breath | 34 (69.4) | 15 (30.6) | ≥ 0.05 |
| General fatigue | 31 (63.3) | 18 (36.7) | ≥ 0.05 |
| Runny nose | 33 (67.3) | 16 (32.7) | ≥ 0.05 |
| Nausea | 36 (73.5) | 13 (26.5) | ≥ 0.05 |
| Cough | 21 (42.9) | 28 (57.1) | ≥ 0.05 |
| Losing taste sense | 38 (77.6) | 11 (22.4) | ≤ 0.05 b |
| Underlying disease | ≤ 0.05 b | ||
| Obesity | 46 (93.9) | 3 (6.1) | |
| Diabetes | 47 (95.9) | 2 (4.1) | |
| Asthma | 46 (93.9) | 3 (6.1) |
a Values are expressed as No. (%).
b P ≤ 0.05 considered statistically significant.
The regression analysis revealed a significant positive relationship (P = 0.001, t = 6.196) between the impact of point mutations in the C-terminal region and the antibody levels in the healthcare staff. Table 4 displays the differences in point mutations in the C-terminal region based on demographic and background variables.
| Sample Code | N-ter (SB) | RBD (SS) | C-ter (VF) | Mutations |
|---|---|---|---|---|
| 5 | × | × | E65Q | |
| 6 | × | × | No mutation | |
| 8 | × | No mutation | ||
| 9 | × | E65Q | ||
| 19 | × | M36I, L37I, K45E, R51X, S61X | ||
| 31 | × | No mutation | ||
| 32 | × | No mutation | ||
| 33 | × | × | S61X, Y33X, S35X | |
| 34 | × | No mutation | ||
| 35 | × | No mutation | ||
| 38 | × | S61X, | ||
| 39 | × | No mutation | ||
| 41 | × | D26N, R29S, K38N, N61K, S98N, E105A |
Mutations were observed in the 25, 10, and 1 sequence of the C-terminal domain (CTD), N-terminal domain (NTD), and RBD (receptor binding domain) regions of the S gene, respectively. Amino acid mutations in the CTD included E65Q, M36I, K45E, R51X, and S61X, while those in the N-terminal region included S35X, Y33X, and S61X. Additionally, amino acid mutations in the RBD region included D26N, R29S, K38N, N61K, S98N, and E105A. Most of the mutations that occurred in the S region of the genome were related to the CTD and can be analyzed and evaluated statistically. Protein sequence analysis of the S protein did not reveal any significant mutations in the virus. Detailed information regarding the mutations is provided in Table 4. The nucleotide sequence data were deposited in the GenBank database under the accession numbers “OR073457.1, OR073473.1, OR073458.1, OR073474.1, OR073475.1, OR073459.1, OR073476.1, OR073460.1, OR073477.1, OR073461.1, OR073478.1, OR073462.1, OR073463.1, OR073479.1, OR073464.1, OR073465.1, OR073468.1, OR073480.1, OR073470.1, OR073481.1, OR073471, OR073472.1, OR073482.1”.
The independent t-test showed that the point mutation in the C-terminal region did not significantly differ based on gender. Furthermore, the ANOVA indicated that the point mutation in the C-terminal region did not significantly differ based on age and vaccine type. According to the conducted ANOVA, there was no significant difference between vaccine types in clinical symptoms such as fever, chills, headache, loss of smell, sore throat, diarrhea, fatigue, shortness of breath, body aches, runny nose, nausea, vomiting, cough, and loss of taste.
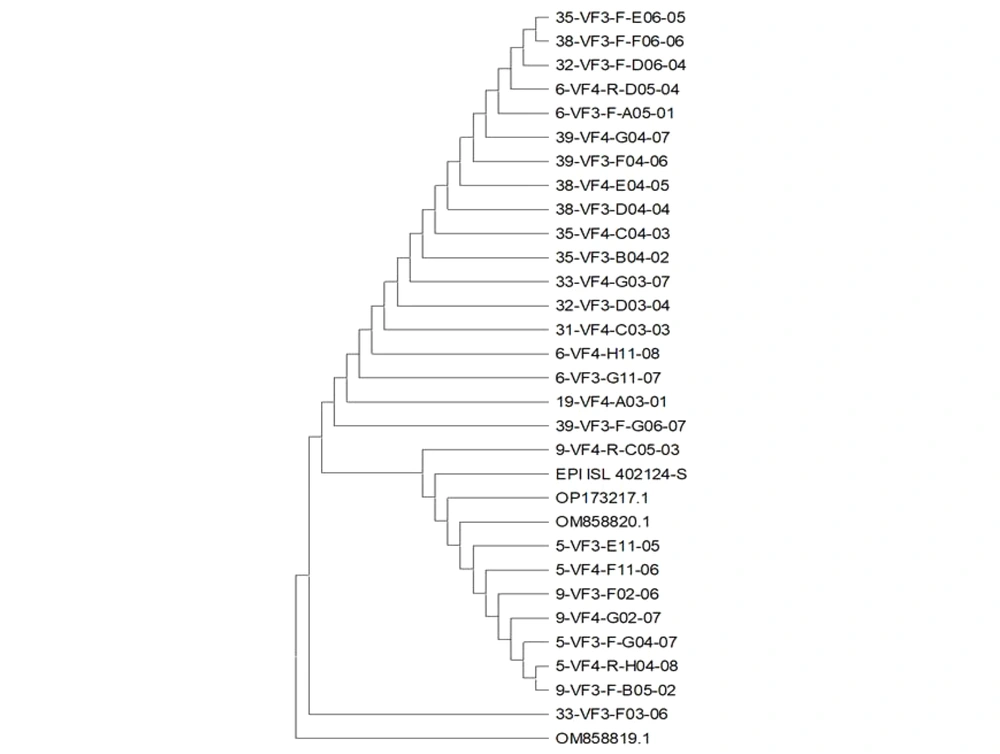
4.1. Phylogenetic Tree
The phylogenetic results of our study on the S gene of SARS-CoV-2 demonstrated similarity with Epi delta-oq414654 corona Iran-om858820 Omicron-NC 045512.2 Wuhan (Figure 1).
5. Discussion
In this study, specific mutations in the breakthrough-infected HCWs have been examined, but no significant mutations were observed in most patients who had received two doses of the vaccine before being infected with COVID-19. These results indicate that the vaccines these individuals received were effective in reducing the development of key mutations in the S-critical region gene, particularly in RBD. On the other hand, vaccination can be effective in reducing the transmission of the Omicron variant and improving the condition of vaccinated individuals who have acquired this variant. Several studies have shown reductions in hospital admissions, mortality, and ICU admissions, in addition to reducing disease severity in people with breakthrough infections (17, 18).
In the current study, certain factors played a role in determining the severity of COVID-19 disease. The most important of these were the use of a ventilator, an oxygen reservoir, the use of vasopressors, and treatment with IVIG or corticosteroids. None of our patients had severe disease, and only 31% of them had mild disease. This study revealed that the severity of the breakthrough infection among HCWs during the COVID-19 Omicron wave was low, which is consistent with other studies (19). Based on the results of this study, it can be said that the vaccines developed against the Omicron variant can still maintain their effectiveness in preventing the disease and reducing its severity in cases of infection.
Several studies have investigated the mutation rate in different regions of the S gene of the SARS-CoV-2 virus among vaccinated individuals. However, there is a lack of specific research examining the mutation rate in the RBD, CTD, and NTD regions of the S gene in vaccinated individuals. One study demonstrated that the mutation rate of SARS-CoV-2 is estimated to be around 1.12 × 10-3 mutations per site per year, which is similar to those observed in the influenza virus and HIV (20). Another study focused on the mutation rate of SARS-CoV-2 during experimental evolution and revealed that the basic mutation rates ranged from 10-6 to 10-3 for various positive-sense RNA viruses (21). Based on the results of this study, it appears that there is a significant and positive relationship between point mutations in the C-terminal region and antibodies in HCWs.
This finding indicates that point mutations in this region may have an impact on the functionality of antibodies against the Omicron variant. It can be suggested that point mutations in the C-terminal region could introduce changes in the protein structure of antibodies, which may lead to a decrease in the capacity of the antibodies to detect and neutralize the Omicron variant (22, 23). In other words, these mutations can potentially reduce the effectiveness of antibodies against the Omicron variant, making individuals with these mutations more susceptible to Omicron infection (24). However, further studies are required to confirm this interpretation and fully elucidate the effect of point mutations in the C-terminal region on antibodies and their responses to the Omicron variant. Additionally, it should be noted that other variables may also influence the effectiveness of antibodies against the Omicron variant, such as antibody levels, individual genetic diversity, and other environmental and educational factors that may play a role in antibody performance (24).
The results of the current study showed that there was no significant difference in the impact of point mutations in the C-terminal region between men and women. This finding indicates that the effect of point mutations in this region on the performance of antibodies against the Omicron variant is comparable between genders. Additionally, the results demonstrated that there was no difference in the impact of point mutations in the C-terminal region based on age and vaccine type. This result suggests that point mutations in this region affect antibody functionality against the Omicron variant similarly across individuals of different ages and vaccine types.
It should be noted that the differences between groups may be influenced by a combination of various factors (25). For example, individuals in different age groups may have received different types of vaccines, which could have an impact on the results (26). Additionally, the presence of underlying diseases in some groups may also contribute to the differences. However, the results showed that there was a difference in the impact of point mutations in the C-terminal region based on the presence of underlying diseases. This finding may indicate that the presence of underlying diseases can influence the severity and functionality of antibodies against the Omicron variant. Furthermore, it is worth mentioning that other factors, such as concurrent infection with other strains of the COVID-19 virus, hygiene conditions, and environmental factors, may also play a role in the impact of point mutations in the C-terminal region (27).
The results of the current study showed that there was no difference in clinical symptoms such as fever, chills, headache, loss of taste and smell, sore throat, diarrhea, fatigue, shortness of breath, body aches, nausea, vomiting, cough, and runny nose among the five types of vaccines: Sinovac, AstraZeneca, Sputnik V, Bharat, and Pfizer. This finding indicates that all five types of vaccines have performed similarly in reducing clinical symptoms of COVID-19, and there is no significant difference among them in this regard. This result is important because it demonstrates that each of these vaccine types is capable of controlling and reducing clinical symptoms of COVID-19.
Individuals who are concerned about these clinical symptoms during vaccination can use any of these vaccine types without having particular concerns about differences in clinical symptoms (28, 29). Gaborieau et al.'s study reported on the prevalence and clinical symptoms of COVID-19. In this study, people with different underlying diseases such as asthma, obesity, and immunodeficiency were also examined, and overall, the most common clinical symptoms in patients were fever, runny nose, digestive problems, and respiratory distress (30). In another study in California, cough, runny nose, and general fatigue were reported as symptoms of patients with COVID-19 (31). Compared to our study. We observed loss of smell sense, sore throat, cough, and fever among the most presented clinical manifestations. Our study includes some limitations, such as the presence of underlying diseases, which may subsequently affect the results. Further investigations on a higher population scale and in other groups within society are needed.
5.1. Conclusions
The study discovered that vaccines developed for the Omicron variant are effective in preventing infections and reducing disease severity. Nevertheless, mutations in the C-terminal region may potentially weaken the antibody response to Omicron. Further research is required to gain a comprehensive understanding of this phenomenon. Gender and vaccine type did not appear to significantly influence these mutations, but underlying health conditions could potentially impact the effectiveness of antibodies. Additionally, all five vaccine types demonstrated similar efficacy in reducing COVID-19 symptoms, implying that any of them can be used effectively against Omicron.