1. Background
Klebsiella species are Gram-negative rods in Enterobacteriaceae family. They can cause different types of infections, such as pneumonia, bloodstream infection, wound or surgical site infections, meningitides, and urinary tract infections (1, 2). Klebsiella spp. has developed resistance to many antibiotics including carbapenems. Carbapenem-resistant K. pneumoniae is the most common species in this genus and it can cause serious infections in healthcare setting and increase mortality rates (1). The recent emergence and dissemination of this resistant species is frequently reported worldwide (3-7). The main reason for carbepenem resistance is the production of carbapenemases with or without high level AmpC or extended-spectrum beta-lactamase (ESBL) production and the loss of porin channel (8, 9).
Carbapenemases are beta-lactamases that hydrolyze penicillins, usually cephalosporins and carbapenems at different levels and monobactams except metallo-beta-lactamases (MBL) (3). The most commonly reported carbapenemases are class A (serine carbapenemases, such as K. pneumoniae carbapenemase (KPC), class B (MBLs, such as New Delhi MBL (NDM), Verona integron-encoded MBL (VIM), and imipenemase (IMP), and class D (OXA carbapenemase such as blaOXA-48 (3, 10). Klebsiella pneumoniae carbapenemase enzymes hydrolyze all beta-lactams and are partially inhibited by clavulanic acid, tazobactam, and boronic acid. Metallo-betalactamases do not hydrolyze aztreonam and their activity is not inhibited by clavulanic acid, tazobactam, and boronic acid, but they can be inhibited by zinc chelators such as ethylenediaminetetraacetic acid (EDTA). On the other hand, blaOXA-48 type enzymes hydrolyze aminopenicillins, ureidopenicillins, and carbapenems, but not significantly hydrolyze the broad-spectrum cephalosporins. Also, they are not inhibited by clavulanic acid, tazobactam, and boronic acid, but their activity is inhibited by NaCl in vitro (11).
Clinical and laboratory standards institute (CLSI) suggests the use of ertapenem as a screening antibiotic and modified Hodge test (MHT) as a confirmatory phenotypic test to detect the carbapenemases (12). Boronic acid and EDTA double or combined disc methods are performed to detect MBLs. Polymerase chain reaction (PCR) is a commonly used method to detect bla genes of carbapenemases (13).
Molecular typing methods determine the clonal relationships between the microorganisms and provide information about epidemiologic data such as the source and transmission routes of the infections, confirmation of outbreaks, and they help to evaluate hospital infection control measures (14, 15). Several molecular methods including plasmid profile analysis, restriction endonuclease analysis (REA) of chromosomal DNA, restriction fragment length polymorphism (RFLPs), random-amplified PCR, ribotyping, pulsed-field gel electrophoresis (PFGE), DNA sequencing, and more recently whole genome sequencing (WGS) are used to assess clonal relatedness of the clinically important bacteria (14, 15). Although WGS has ultimate resolution and is already used for the molecular typing of many microorganisms in developed countries, it is still too laborious and time-consuming to obtain useful data in routine surveillance (15). Currently, among the DNA fingerprinting methods, PFGE is a worldwide used reliable, discriminative, and reproducible method for a variety of bacterial species including K. pneumoniae (16-18).
2. Objectives
The infections caused by carbapenem-resistant K. pneumoniae isolates are a current challenge during treatment and infection control in hospitals due to their rapid transmission and the limited antibiotic options available. Identification of clonal relationship and resistance mechanisms among these resistant strains isolated from hospitalized patients would be very important to evaluate effectiveness of the infection control and treatment programs applied in the study population and develop more effective programs to minimize spread of the resistant pathogens. Therefore, the current study aimed at investigating the carbapenemase enzymes and clonal relationships among the carbapenem-resistant K. pneumoniae isolates in a tertiary hospital.
3. Methods
3.1. Ethics Statement
The current study protocol was approved by the ethics committee of Ankara Ataturk training and educating hospital (date, 21/05/2014, number 78). The study was conducted in accordance with the tenets of the Helsinki declaration. The current study was carried out in a tertiary training and research hospital that had 488 beds with 10 intensive care units (ICUs) from March 2012 to January 2015.
3.2. Bacterial Isolates
Klebsiella pneumoniae isolates were cultured from various clinical samples including blood, urine, wound/abscess, tracheal aspirate/bronchoalveolar lavage, and rectal swabs of the inpatients. Biochemical tests such as the fermentation of glucose and lactose, hydrogen sulfide production, citrate utilization, urease activity, motility, indol production, ornithine-decarboxylase activity, and commercial API 20E (Bio-Merieux, France) kit were used to identify K. pneumoniae isolates.
3.3. Antibiotic Susceptibility Testing
All identified K. pneumoniae isolates were subjected to antibiotic susceptibility testing by Kirby-Bauer disc diffusion method and the results were interpreted according to CLSI criteria (12). As recommended by CLSI, Escherichia coli ATCC 25922 strain was used as a negative control. Ertapenem was used to screen carbapenem resistance as recommended by CLSI. Ertapenem resistance was confirmed by the gradient diffusion method (E-test, Bio-Merieux, France).
3.4. Modified Hodge Test
The MHT was performed for all ertapenem-resistant isolates by following the protocol of CLSI (12). Briefly, a 0.5 McFarland standard suspension of E. coli ATCC 25922 was prepared in saline. A 1:10 dilution of this suspension was streaked as lawn onto a Mueller-Hinton agar (MHA) plate. Ertapenem disc (10 µg) was placed in the center of the plate. Test and control samples were inoculated in a straight line from the edge of ertapenem disc to the edge of the plate. After 18 to 24 hour incubation, the plates were examined for the enhanced growth around the bacteria straight at the intersection of the straight line and the zone of inhibition. The presence of enhanced growth was evaluated as positive for carbapenemase production. K. pneumoniae ATCC-BAA1705 and K. pneumoniae ATCC-BAA1706 were used as positive and negative controls, respectively.
3.5. Ertapenem/Ethylenediaminetetraacetic Acid-Combined Disk Test
Ertapenem/EDTA-combined disk test (Ertapenem-ECD) was performed to detect metallo-β-lactamases. A 0.5 McFarland suspension of test samples was inoculated onto plates with MHA. Two ertapenem discs (10 μg) were placed on the plate, and 10 μL of EDTA solution was added to one of them to obtain the desired EDTA concentration (750 μg). The inhibition zones of the ertapenem and ertapenem/EDTA disks were compared after 16 to 18 hours of incubation. If the increase in inhibition zone with the ertapenem and EDTA disc was ≥ 7 mm as compared with the ertapenem disc alone, the isolate was considered as MBL positive (13, 19).
3.6. Polymerase Chain Reaction
All carbapenem-resistant K. pneumoniae isolates were tested for the presence of carbapenemase-encoding genes including blaOXA-48, blaNDM-1, blaKPC, blaIMP, and blaVIM by the multiplex PCR using the primers described previously (20-22). A bacterial suspension (equal to McFarland 4 turbidity) was prepared in sterile water and boiled for 10 minutes. Then, it was centrifuged for 2 minutes at 13000 g and the supernatant was used as the DNA source. The PCR reaction mixture contained 1.5 mM MgCl2, each of the deoxynucleotide triphosphates at a concentration of 200 μM, 5 μL of 10 × PCR buffer (750 mM Tris-HCl (pH 8.8), 200 mM (NH4)2SO4, 0.1% (v/v) Tween 20), 10 pmol of each primer, and 2.5 U of Taq DNA polymerase (Thermo Scientific-Fermantas Corporation, Vilnius, Lithuania) in a final reaction volume of 50 μL. The following amplification conditions were used: initial denaturation at 94°C for 3 minutes, followed by 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute, with a final extension step at 72°C for 7 minutes. The amplified DNA products were analyzed by electrophoresis on a 1.5% agarose gel and the results were evaluated according to the size of each amplicon (20-22).
3.7. Pulsed-Field Gel Electrophoresis
Pulsed-field gel electrophoresis (PFGE) of the carbapenem-resistant K. pnemoniae isolates was performed following the protocol of Durmaz et al. (23). Briefly, bacterial cells were embedded into low melting agarose including sodium dodecyl sulfate. Cells in plugs were digested with lysozyme and proteinase K. After washing the plugs, genomic DNA was digested with 20 U of XbaI restriction endonuclease (Thermo Scientific-Fermantas Corporation, Vilnius, Lithuania). DNA fragments were separated using a CHEF-DR II system (Bio-Rad Laboratories, Nazareth, Belgium) at 14°C with 6 Volt/cm2 for 20 hours. The initial and final switching time was 5 and 30 seconds, respectively. The gel was stained with ethidium bromide (5 µg/mL) for 20 minutes and destained with distilled water for 30 minutes. The DNA band profiles were visualized under UV light, photographed using gel logic 2200 imaging system (Kodak Co., Rochester, NY, USA), and analyzed by GelCompar II electrophoresis software (version 7.0; Applied Maths, Sint-Martens-Latem, Belgium). A 1% band tolerance was used to compare DNA profiles. Clonal relationship among isolates was evaluated by the criteria of Tenover et al. (16).
3.8. Statistical Analysis
The data were analyzed with SPSS software version 18. The Pearson chi-square test was used to analyze the data and statistically significant level was accepted as less than 0.05.
4. Results
Ertapenem resistance was determined in 93 of the 1605 (5.8%) K. pneumoniae isolates cultured from various clinical samples and rectal swabs of the inpatients. Of the carbapenem-resistant K. pneumoniae isolates, 28 (30.1%) were isolated from rectal swab samples of the inpatients and these patients were evaluated as colonized with K. pneumoniae. The remaining 65 carbapenem-resistant K. pneumoniae isolates were recovered as a pure culture from blood (n = 20, 21.5%), urine (n = 20, 21.5%), wound/abscess (n = 17, 18.3%), and tracheal aspirate/bronchoalveolar lavage (n = 8, 8.6%) of the inpatients who had clinical signs of pneumonia, sepsis, urinary tract infections, abscess, or burn wound infections. These clinical and laboratory findings were evaluated together and these isolates were accepted as infectious agents. The 71% (66/93) of the resistant isolates were from ICUs, others were isolated from the patients hospitalized in various wards (table 1 in Supplementary file).
Ninety-one (97.8%) of the carbapenem-resistant K. pneumoniae isolates were positive with MHT conforming carbapenemase production. Only 6 (6.5%) isolates were positive with ECD method demonstrating MBLs (table 1 in Supplementary file). Multiplex PCR yielded negative results in 3 (3.2%) of the 93 carbapenem-resistant isolates, the remaining 90 isolates were positive. All 6 isolates showing ECD positivity had blaNDM-1 (6.5%). The majority of the resistant isolates had blaOXA-48 (n = 84, 90.3%). No isolate had both blaNDM1 and blaOXA-48 simultaneously. The blaKPC, blaIMP, and blaVIM resistance genes were not detected in any of the isolates (table 1 in Supplementary file).
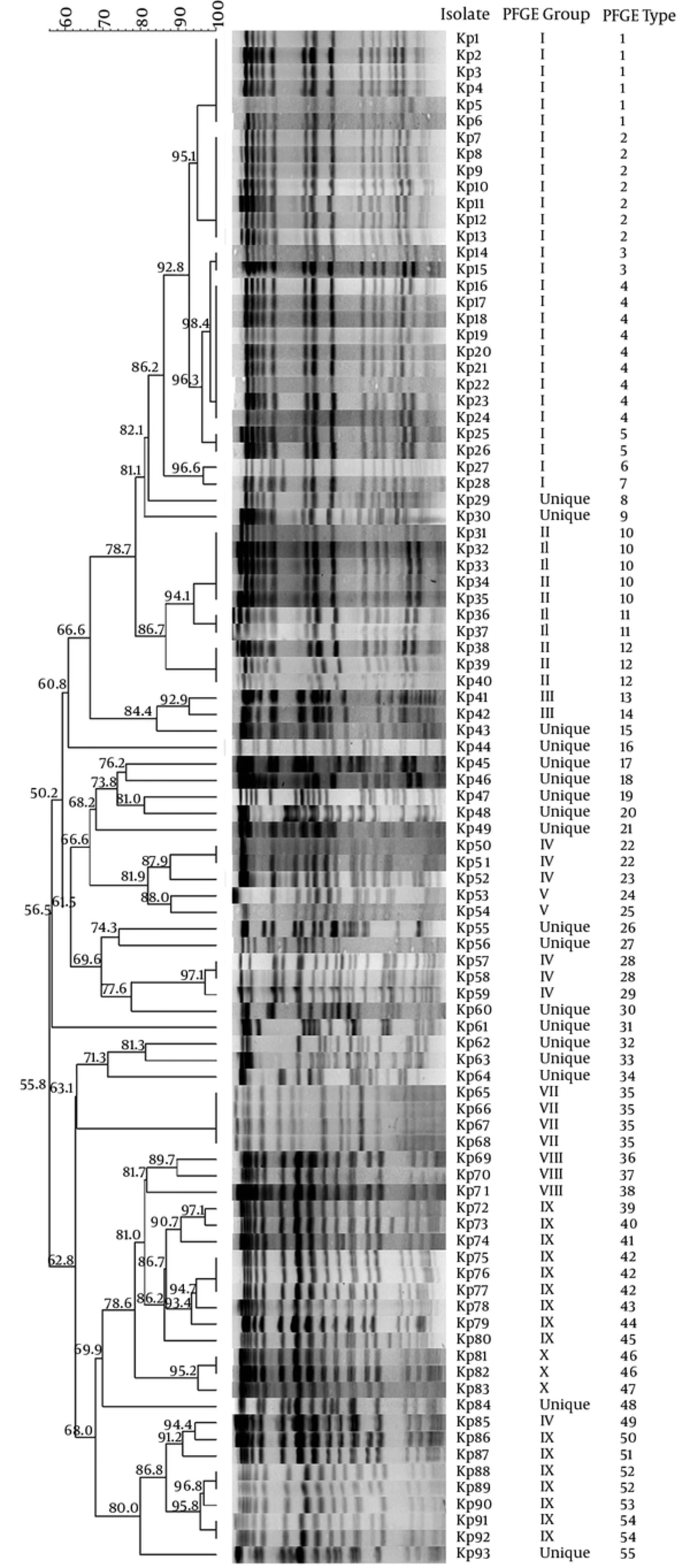
Pulse-field gel electrophoresis of the 93 K. pnemoniae isolates yielded 55 different pulsotypes (PFGE types). Of the pulsotypes, 15 comprised of clusters and the size of each ranged from 2 to 9 strains, while the remaining 40 were unique, each included 1 strain only. The clustering rate was 57% (53/93 isolates). Based on the application of similarity coefficient higher than 85%, 11 major PFGE groups were distinguished and 80.6% of the tested strains were clonally related. Pulsotype 4 was the largest one, harboring 9 strains, followed by pulsotype 2 (seven strains), pulsotype 1 (six strains), pulsotype 10 (five strains), and pulsotype 35 (four strains) (Figure 1).
The isolates with indistinguishable PFGE types were indicated with the same number. The isolates showing PFGE types, which differed from the other only one or more bands, were indicated with unique numbers. PFGE groups indicated with the same Roman numbers were defined based on the similarity coefficient ≥ 85%. The numbers given on the branches indicated similarity coefficient values.
The 66 K. pnemoniae isolates cultured from the patients in ICUs were classified in 40 different pulsotypes. The 12 pulsotypes were clusters including a total of 38 isolates; the clustering rate was 57.6%. On the other hand, 21 different pulsotypes were determined among the 27 isolates from various services and the clustering rate was 37%. There was no significant difference between the clustering rates of the isolates from the ICUs and those of the other clinics (P = 0.072). According to the clinical samples, there was significant difference between the clustering rates of the isolates cultured from different samples (P = 0.006). The highest clustering rate was observed in the rectal swab isolates (64.3%), followed by urine (40%), blood (35%), and wound/abscess (23.5%) isolates. All lower respiratory tract isolates showed unique pulsotypes (Table 2).
| Type of Sample | No of Isolates | PFGE Typing Results | |
|---|---|---|---|
| No. of different pulsotypes | Clustering rate, % | ||
| Blood | 20 | 16 | 35 |
| Urine | 20 | 16 | 40 |
| Wound/abscess | 17 | 14 | 23,5 |
| Lower respiratory tract | 8 | 8 | All unique |
| Rectal swab | 28 | 16 | 64.3 |
The PFGE Typing Results of K. pneumoniae Isolates in Different Samples
5. Discussion
There is a real threat of the increasing carbapenemase producing isolates that causes serious hospital-acquired infections and the high mortality rates. The prevalence of carbapenem resistance in K. pneumoniae isolates was 3.6% to 10.8% in USA according to the center for disease control and prevention (CDC) 2008 report. Meanwhile in Europe, according to European antimicrobial resistance surveillance network (EARS-Net) data, it was reported as 7.3% (24, 25). In Turkey, the rate of carbepenem resistance was reported in 2.2% of K. pneumoniae isolates recovered in a university hospital from 2004 to 2007 (26). Thereafter, the increased resistance rates varying from 3.7% to 16.6% were reported from different hospitals (27-29).
In the Hitit-2 surveillance study of 2007 performed on the isolates from 13 hospitals located in different geographic regions of Turkey, the prevalence of carbapenem resistance in K. pneumoniae was notified as 3.1% ranging from 0 to 16% (27). Similarly, Kuzucu et al. found 3.6% carbapenem resistance among the K. pneumoniae isolates from the East part of Turkey in the same time period (28). Then, the rate of carbapenem resistance slightly increased to 6.8% in 2013 (29). In agreement with the previous data, the current study showed that 5.8% of K. pneumoniae isolates recovered from a tertiary hospital in the capital city of Turkey were phenotypically resistant to carbapenems.
Modified Hodge test is a reliable test especially for KPC and blaOXA-48 producing isolates, but not for MBL producers (12). Similar to the current study data, the current study found that nearly all ertapenem-resistant K. pneumoniae isolates with blaOXA-48 were positive with MHT. Parallel to the current study data, Kuzucu et al., also showed that all of the carbapenem-resistant isolates were MHT positive (28). In contrast to these results, in another study, MHT positivity was found only in 1 out of 7 isolates with carbapenemase genes (5), and it was concluded that MHT might be false negative, especially in the presence of ESBL or AmpC with reduced porine activity (5, 30). In the present study, ECD method was positive in 6.5% of the tested ertapenem-resistant isolates and all of them harbored blaNDM-1, suggesting high concordance between ECD method and PCR. This result was in agreement with those of the previous reports indicating the reliability of combine disc methods to detect MBL producing isolates (30, 31).
In the present study, more than 90% of the carbepenem-resistant K. pneumoniae isolates were blaOXA-48 positive. This enzyme was firstly reported from a K. pneumoniae isolate in Turkey in 2001 (22). Later, it was reported from different hospitals with increased rates (29, 32). A multi-central surveillance study performed at a Turkish university hospital from 2009 to 2013 showed that more than 96% of K. pneumoniae isolates harbored blaOXA-48 carbapenamese (21). In another multi-central study conducted on the patients from different hospitals of Turkey as part of the European survey of carbapenemase producing Enterobacteriaceae (EuSCAPE), the high prevalence (83%) of blaOXA-48 was also confirmed (33).
In the current study, blaNDM-1 gene was observed in 6 (6.5%) of the resistant isolates, 4 of them were from clinical samples and 2 from rectal swabs. There were no direct epidemiological link such as common isolation date and/or hospitalized wards between these isolates, except that 2 of them were in the same pulsotype, suggesting that the NDM-1 producer isolates mostly came from different clones. In Turkey, the first NDM-1 positive K. pneumoniae isolate was reported from the blood culture of a child patient in 2011 (34). In 2013, the study performed on 94 carbapenem-resistant K. pneumoniae isolates recovered from rectal swab samples of inpatients reported that 4.3% of the isolates produced only NDM-1, and 1% produced both blaOXA-48 and NDM-1 (35). Currently, a NDM-1 positive K. pneumoniae epidemic was described in a tertiary hospital and 2 of the isolates also harbored the blaOXA-48 (36). Parallel to the results of Turkey, the emergence of NDM-1 producers among K. pneumonae and other Enterobacteriaceae members is reported worldwide, especially from India and Middle East and Far East countries (37, 38).
Verona integron-encoded MBLs and IMPs are the most common MBLs worldwide especially in Greece, Taiwan, and Japan. Klebsiella pneumoniae carbapenemase was first reported in 1996, and became more widespread and today is the most common carbapenemase (3, 7, 39). In the present study, KPC, IMP, and VIM were not determined from any of the isolates. Similar to the current study data, 2 recent studies performed on carbapenem-resistant K. pneumoniae clinical isolates from different hospitals of Turkey, no positive results for KPC, IMP, and VIM enzymes were reported (40, 41). In a multicentral surveillance study, the prevalence of VIM, IMP, and KPC among 136 carbapenem-resistant E. coli and K. pneumoniae isolates was reported as 2.8%, 1.4%, and 0%, respectively (33). Based on the results of the current and those of previous studies, it can be speculated that these enzymes are not a major problem in Turkey yet; however, it is worth to follow their incidence.
Pulse-field gel electrophoresis provides very useful data to understand the epidemiology of carbapenem-resistant K. pneumoniae isolates and to revise and enhance infection control programs to prevent bacterial transmission (17). In the current study, the results of PFGE showed that more than half of the carbapenem-resistant K. pneumoniae isolates were identified in clusters and according to the similarity coefficient higher than 85%, clonally relatedness among the tested isolates increased to 80.6%. This result indicated the emergence and spread of these resistant isolates across the hospital. The current study also indicated that cross-transmission was not restricted in a specific clinic or time period. For instance, although the 9 K. pneumoniae isolates clustered in the largest PFGE type, 4 were mainly isolated from the reanimation unit, this clone was also found in 2 other services and it stayed in the studies hospital in a 20-month period. The second largest PFGE type 2 comprised of 7 isolates collected from 4 different samples of the patients in 5 different services during the 6-month period.
The PFGE type 1 included 6 species isolated from the patients hospitalized in 4 different services during a period of 12 months. Similar to the current study, PFGE of multidrug-resistant K. pneumoniae isolates in Egypt revealed that clonally related strains were isolated from various sources such as different patients in the same hospital and environmental samples (17). A recent study performed on carbepenem-resistant K. pneumoniae clinical isolates in Shandog, China, showed that the strains in the same PFGE type were isolated in the same clinic for more than 2 years (6). As already indicated (18, 42), the high rate of clonally relatedness among the isolates from different services in a wide time period was an important evidence for ongoing cross-transmission of these resistant isolates in the current study setting. Infected or colonized patients, hospital staff, and/or contaminated equipment might affect this cross-transmission.
5.1. Conclusion
High rate (80.6%) of clonal relationship between the carbapenem-resistant isolates collected in a 3-year period indicates a longtime cross-contamination in study population. Therefore, strict infection control and surveillance measures combined with careful and reasonable use of antibiotics are very important to minimize the spread of carbapenem resistance.