1. Background
The COVID-19 pandemic, triggered by the SARS-CoV-2 virus, has become a significant global health challenge, causing widespread morbidity and mortality (1). The primary symptoms associated with COVID-19 encompass fever, cough, shortness of breath, muscle aches, and fatigue (2), while less common symptoms may include sputum production, headaches, hemoptysis, and diarrhea. In its severe form, the disease can escalate to infectious pneumonia, potentially leading to acute respiratory distress syndrome (ARDS), cardiac damage, and secondary infections (3). Despite concerted global efforts to curb the spread of this highly contagious virus, there remains an urgent demand for effective diagnostic tools and prognostic markers to better manage and treat those affected.
Numerous studies have indicated that individuals with COVID-19 show increased levels of proinflammatory cytokines in their blood (4). In certain instances, the disease may advance swiftly, resulting in a condition known as a cytokine storm. This condition overwhelms the immune system, leading to a significant decrease in lymphocytes, particularly T cells (5). The disruption of the immune system's overall functionality causes immune cells to release an excessive amount of pro-inflammatory cytokines and chemokines, exacerbating the cytokine storm (6). The overwhelming inflammation induced by the virus in COVID-19 patients is closely linked to the disease's severity and has been identified as a leading cause of death (7).
Chemokines, as inflammatory mediators, are essential in aiding the immune response to infections. Yet, their overproduction is a key factor in hyperinflammation (8). A meta-analysis has shown that severe COVID-19 cases present higher levels of chemokines compared to moderate cases (9). Biomarkers like IP-10 and MCP-1 are associated with the severity of COVID-19 and the mortality risk among those infected (10). Current diagnostic approaches for COVID-19 fall short in identifying mild or asymptomatic cases and in forecasting the progression of the disease.
2. Objectives
This study was conducted to assess the circulating levels of monocyte chemoattractant protein 1 (MCP-1), programmed death protein 1 (PD-1), interleukin 8 (IL-8), and tumor necrosis factor α (TNF-α) as chemokines and cytokines in individuals with severe COVID-19, in comparison to those with mild cases and individuals who have recovered from the disease. These biomarkers are key players in modulating the immune response, cytokine signaling, and inflammation, which are critical factors in the pathogenesis of COVID-19. The identification of reliable biomarkers is essential for the early detection of the disease, risk assessment, and informing treatment strategies.
3. Methods
In this case-control study, carried out between 2019 and 2021, participants were selected from Masih Daneshvari Hospital. The research involved groups of individuals diagnosed with severe COVID-19, those with mild COVID-19, and individuals who had recovered from the disease, with 40 participants in each category (Table 1). The SARS-CoV-2 infection in all participants was verified through viral PCR testing of nasal and pharyngeal swab specimens obtained during the acute phase of the infection, following WHO guidelines.
| Disease Severity | Specifications |
|---|---|
| Mild | Mild clinical signs (fever above 38°C, with or without cough, no dyspnea), no visible sign of pneumonia |
| Medium | Fever; Respiratory symptoms, the visible sign of pneumonia |
| Severe | Respiratory distress, respiratory rate (RR) more than 30 times per minute-pulse oximetry oxygen saturation level (SPO2) less than 93% at rest- The arterial partial pressure of O2 and the fraction of inspired oxygen (PaO2/FiO2) ratio less than 300 mmHg |
3.1. Enzyme-linked Immunosorbent Assay
Peripheral blood samples were collected from each participant post-diagnosis by a healthcare specialist through standard venipuncture. Serum samples were separated via centrifugation at 1000×g for 15 minutes at 4°C and were then promptly stored at -80°C pending further analysis. The concentrations of soluble IL-8 and TNF-α were quantified by Enzyme-linked Immunosorbent assay (ELISA), following the instructions provided by the kit's manufacturer. The optical density (OD) for IL-8 and TNF-α (both supplied by Carmania Parsgen Co, Iran) was determined spectrophotometrically using a microplate reader at wavelengths of 450 nm and 620 nm, respectively. The quantities of IL-8 and TNF-α were established based on a standard curve.
3.2. qRT-PCR
RNA was extracted from peripheral blood samples utilizing the RNeasy Midi Kit (Qiagen, Cat No.75144) following the manufacturer's guidelines. Subsequently, cDNA was synthesized from the extracted RNA using the Viva 2-step qRT-PCR Kit (Cat No. RTPL12). The expression levels of MCP-1 and PD-1 were quantified via qRT-PCR using CinnaGreen qRT-PCR Mix, 2X (Cat No.MM2041), with 18srRNA serving as the internal control for normalization, as per the manufacturer's instructions. The gene expression data were normalized against 18srRNA and analyzed using the ΔΔCt (2−ΔΔCt) method. The specific primer sequences employed in this expression study are detailed in Table 2.
| Parameters | MCP-1 | PD-1 | 18S rRNA |
|---|---|---|---|
| Forward primer | AGAATCACCAGCAGCAAGTGTCC | CGTGGCCTATCCACTCCTCA | GTAACCCGTTGAACCCCATT |
| Reverse primer | TCCTGAACCCACTTCTGCTTGG | ATCCCTTGTCCCAGCCACTC | CCATCCAATCGGTAGTAGCG |
| Length of amplified fragment | 98 | 106 | 152 |
a The number of each gene, sequence, size and annealing temperature is specified separately.
3.3. Statistical Analysis
Statistical analyses were conducted using SPSS 24 software. Group differences were assessed using a one-way ANOVA test followed by Tukey's HSD post-hoc analysis. The correlation between variables was examined using Spearman's rank correlation coefficient. The area under the receiver operating characteristics (ROC) curve (AUC) was calculated for the patients, and the optimal cut-off value was identified using Youden’s index. All results are reported as mean ± standard deviation (SD), with P-values of less than 0.05 deemed statistically significant.
4. Results
4.1. Patient Characteristics
The study included 120 individuals diagnosed with COVID-19, categorized into three distinct groups based on the severity of their condition: Severe, mild, and recovered. There were no significant variations in age across these groups. The average ages SD for patients with severe, mild, and recovered COVID-19 were 55.62 (8.22), 54.48 (7.94), and 55.32 (8.88) years, respectively, with statistical analysis indicating no significant differences in age among the groups (P = 0.41). The distribution of males in the severe, mild, and recovered groups was 60%, 62%, and 58%, respectively, showing no significant differences in gender distribution across the groups (P = 0.07).
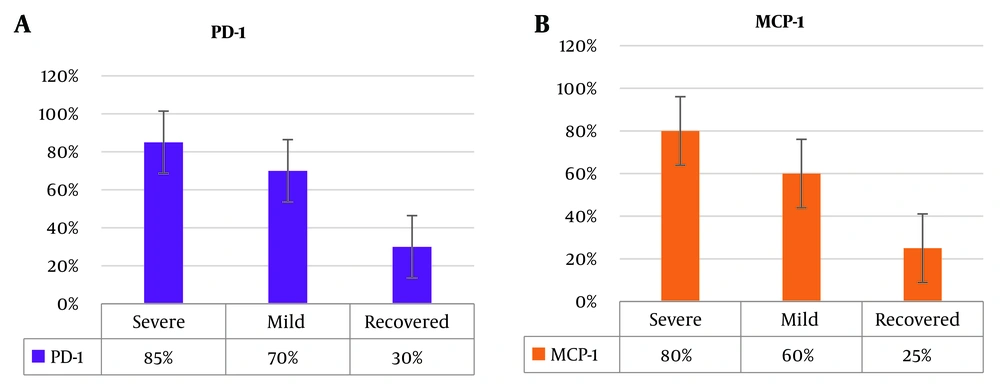
The biomarkers IL-8, TNF-α, PD-1, and MCP-1 exhibited varying positivity rates across different infection severity groups. Specifically, in the severe infection group, the positivity rates for IL-8, TNF-α, PD-1, and MCP-1 were 92.00%, 90%, 85%, and 80%, respectively. The corresponding positivity rates for the mild infection group were 75%, 78%, 70%, and 60%. In the recovered group, the rates of positive results for IL-8, TNF-α, PD-1, and MCP-1 stood at 28%, 22%, 30%, and 25%, respectively, as depicted in Figure 1. A statistical analysis comparing the positivity rates of these biomarkers among the severe infection, mild infection, and recovered groups, conducted using the two-sample binomial test, indicated a statistically significant difference across the groups (P < 0.001).
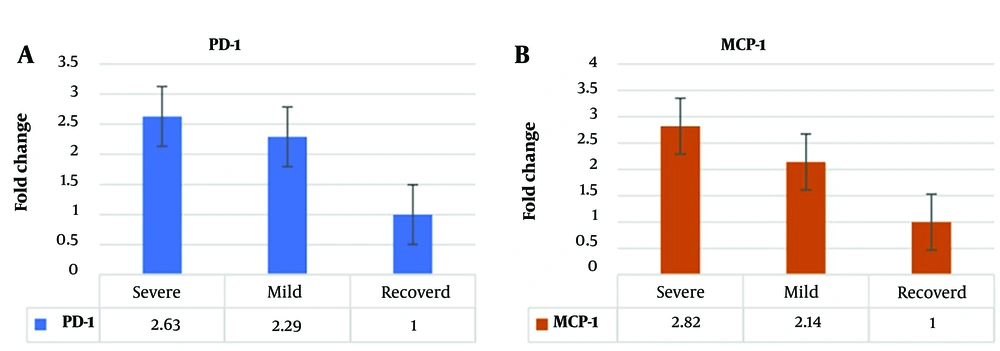
The expression level of PD-1 in individuals with severe infection was significantly elevated, showing a 2.63-fold increase when compared to healthy individuals. In the case of individuals with mild infection, PD-1 levels were increased by 2.29-fold relative to healthy individuals, as depicted in Figure 2. Similarly, the expression level of MCP-1 in those with severe infection was 2.82 times higher than in healthy individuals. MCP-1 levels were 2.14 times higher for individuals with mild infection than those in healthy individuals, as shown in Figure 2. These findings highlight significant differences in the expression levels of PD-1 and MCP-1 across different infection severity groups, underscoring their potential utility as valuable biomarkers for determining the severity of infection.
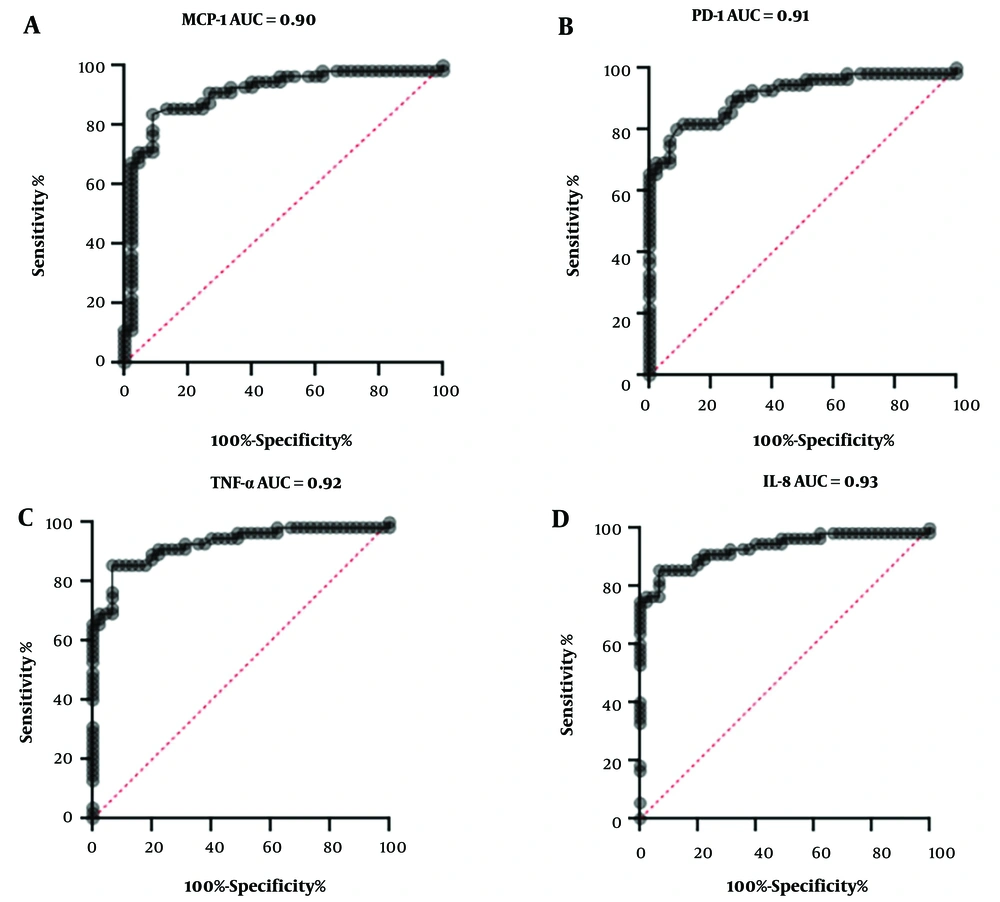
We evaluated the correlation to determine any association between cytokines/chemokines and disease severity. A strong positive linear relationship was observed between PD-1 and MCP-1 in patients with severe infections (r = 0.64, P < 0.05), indicating a significant association. However, no correlation was found between PD-1 and MCP-1 in patients with mild and recovered COVID-19 infections. Similarly, no correlation was observed between IL-8 and TNF-α across severe, mild, and recovered COVID-19 infection groups. The diagnostic utility of cytokines and chemokines in determining illness severity was further analyzed using the receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC) as shown in Figure 3. The AUC for ROC values for MCP-1, PD-1, TNF-α, and IL-8 were 0.90, 0.91, 0.92, and 0.93, respectively, suggesting that these cytokines could effectively predict the severity of COVID-19 infections (Figure 3). Our data suggest that these cytokines and chemokines may be biomarkers for disease severity in COVID-19-infected patients.
5. Discussion
COVID-19 manifests in a spectrum ranging from asymptomatic cases and spontaneous recovery to severe ARDS, characterized by respiratory failure and lung damage. Identifying individuals at high risk of adverse outcomes and pinpointing potential risk factors and biomarkers for severe illness is crucial. To this end, we utilized ELISA and qRT-PCR analyses on the serum of COVID-19 patients. Our findings indicated that patients with severe infections exhibited a higher percentage of positive results for IL-8, TNF-α, PD-1, and MCP-1 compared to those with mild infections or those who had recovered. Subsequent statistical analysis using ROC curves suggested that these cytokines and chemokines could serve as important indicators for assessing the severity of COVID-19 in affected individuals.
Numerous research studies have indicated that patients with severe cases of COVID-19 often exhibit significant increases in the levels of proinflammatory cytokines in their blood (6, 11). While the exact mechanisms of how cytokines communicate in COVID-19 patients remain largely unknown, there have been growing achievements in utilizing cytokines or cytokine inhibitors as therapeutic interventions (12, 13). Interleukin 8 is a chemokine released by various cell types, including endothelial cells, epithelial cells, macrophages, and monocytes. It acts on leukocytes such as neutrophils and natural killer cells, inducing chemotaxis, degranulation, and the production of reactive oxygen species (14). The accumulation of activated neutrophils, which can cause damage to lung tissue, has been proposed as a mechanism contributing to ARDS (14).
Elevated levels of IL-8 in the bloodstream have been linked to a longer duration of illness in patients with severe or critical COVID-19 (15). IL-8 has also been associated with the recruitment and activation of polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSC), which inhibit the response of T-cells to the SARS-CoV-2 virus (16). Furthermore, the frequency of PMN-MDSCs is higher in non-survivors compared to survivors of critical COVID-19, and their frequency is positively correlated with plasma levels of IL-8 in hospitalized COVID-19 patients (16). These findings suggest that IL-8 signaling plays a crucial role in the disease progression, revealing potential therapeutic strategies for treating COVID-19 (17).
Our findings demonstrated that severe COVID-19 patients had higher levels of MCP-1 in their peripheral blood cells than those with mild symptoms or who had recovered. Previous studies on severe cases of SARS-CoV have also shown that elevated MCP-1 secretion is associated with lung injury and the need for intensive care unit (ICU) admission (6, 18-20). Additionally, MCP-1 is a parameter linked to thrombosis, which suggests it may be associated with an increased risk of mortality in COVID-19 patients (20). Consistent with our results, Huang et al. reported a significant increase in serum levels of proinflammatory cytokines, particularly MCP-1, in patients infected with 2019-nCoV, indicating its potential role in activating the T-helper-1 (Th1) cell response.
We found that serum tumor necrosis factor α (TNF-α) levels were elevated in COVID-19 patients with severe disease and adverse prognosis. This finding aligns with previous research indicating that cytokine release syndrome (CRS) is strongly associated with disease severity, characterized by increased serum levels of TNF-α, granulocyte-colony stimulating factor (G-CSF), interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1α (MIP1A) (6, 21). An increase in TNF-α can potentially facilitate viral infection and cause damage to organs (22). While TNF-α plays a role in coordinating the inflammatory response during the acute phase of inflammation, excessive TNF-α can suppress the immune system, leading to adverse outcomes (23, 24).
Moreover, upregulation of PD-1 in T-cells was detected during the progression of symptomatic stages of COVID-19 (25). This upregulation of PD-1 has been found to contribute to developing the severe form of the disease (26). Consistent with this, our study revealed an increased level of PD-1 in patients experiencing severe symptoms, suggesting a potential association between PD-1 and the severity of COVID-19. SARS-CoV-2 triggers lymphocyte depletion and exhaustion in infected individuals. This is primarily characterized by the expression of programmed death inhibitory receptor 1 (PD-1) and programmed death ligand 1 (PDL-1) (27, 28), which play crucial roles in central and peripheral immune tolerance and exhaustion.
Regarding the correlation analysis, a strong positive relationship was observed between PD-1 and MCP-1 in severe COVID-19 patients. Studies have indicated that COVID-19 patients exhibit increased expressions of PD-1 in both CD4+ and CD8+ T cells. This suggests the involvement of these regulatory molecules in the apoptosis of antigen-activated T cells during COVID-19, resulting in a decrease in CD4+ T cell numbers and a reduction in the proportion of naive T cells (29). On the other hand, there is evidence indicating a rise in the population of monocytes, neutrophils, and natural killer (NK) cells, triggering a cytokine storm (30, 31). Additionally, MCP-1 is implicated in the pathogenesis of diseases characterized by the infiltration of monocytes (32). Based on these findings, it is plausible to speculate that MCP-1 and PD-1 may be associated with the severity of COVID-19.
5.1. Conclusions
The study has limitations due to a small sample size and the lack of analysis of inflammatory mediators over time. More multi-center studies are needed to verify the results and provide a comprehensive interpretation. However, the study supports the association of IL-8, TNF-α, PD-1, and MCP-1 with the severity of COVID-19. It is important to understand the specific functions of these cytokines and evaluate treatment consequences before considering modulating therapy. Our data suggest that modulators of elevated cytokines/chemokines may offer potential precision medical treatments for COVID-19. The results suggest that MCP-1, PD-1, TNF-α, and IL-8 may be biomarkers for disease severity in COVID-19-infected patients. These findings are expected to enhance our comprehension of the immunopathological mechanisms associated with this emerging disease.