1. Background
Similar to severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1), the viral entry into target cells by SARS-CoV-2 is the mediated interaction between the viral spike (S) protein and cellular angiotensin-converting enzyme 2 (ACE2); nevertheless, SARS-CoV-2 is bound more efficiently to ACE2 than SARS-CoV-1, increasing its damaging pathogenicity and its ability for transmission (1). Severe acute respiratory syndrome coronavirus 2 utilizes the ACE2 receptor for cellular entry, making cells with high ACE2 expression susceptible to infection and damage. This receptor is expressed in various organs, including the small intestine, kidneys, and heart, which could potentially be targeted by the virus, leading to organ failure in some cases (2, 3). Numerous studies have demonstrated elevated ACE2 expression in the human testis (4-6). Moreover, recent reports have documented the presence of SARS-CoV-2 ribonucleic acid (RNA) in various clinical specimens, including semen (7, 8). The aforementioned findings have prompted several critical questions: (1) Can SARS-CoV-2 infect the testis through the ACE2 receptor, potentially disrupting spermatogenesis?; (2) Does sexual transmission via seminal shedding pose a risk of viral spread?; (3) Could SARS-CoV-2 infection in males have adverse reproductive implications, particularly for young men pursuing parenthood?
It was reported that SARS-CoV-2 could be detected in patients’ semen from mild to moderate symptoms or recovery of coronavirus disease 2019 (COVID-19) and might induce epididymal-orchitis and negatively affect spermatozoa and male fertility (9). Li et al. detected SARS-CoV-2 genomic sequence within the semen samples of six patients with COVID-19 (8), and Yang et al. (10) identified SARS-CoV-2 RNA in the testicular tissue of one patient (1/12) with a high-level viral load in the upper respiratory tract. However, the findings of several studies do not support the previous research, and SARS-CoV-2 RNAs were not detected in patients’ semen samples with acute symptoms of/or recovering from COVID-19 (11-13).
In the aforementioned studies, as most of the semen samples came from patients in the recovery stage, the virus might have cleared up during detection time; therefore, the existence of SARS-CoV-2 in the semen was not ruled out. Nevertheless, although SARS-CoV-2 shedding and viral entry into semen is still elusive, based on some studies, patients with a moderate infection had a significant impairment of semen quality. Moreover, a recent systematic review and meta-analysis has revealed that the mean duration of SARS-CoV-2 RNA shedding is 16.6 days in serum samples, 17 days in the upper respiratory tract, and 17.2 days in the stool. With the passage of days after infection, it has been hypothesized that there is a shifting of RNA positivity from throat swabs to various body fluids (14).
Despite the resolution of clinical symptoms within the first week of infection, SARS-CoV-2 RNA can persist in pharyngeal swabs for up to 2 weeks. Similarly, viral RNA has been detected in stool and sputum samples for up to 3 weeks after clinical recovery. This extended shedding pattern is also observed in semen (15, 16). Despite extensive research on COVID-19, several key questions remain regarding the virus's mechanisms, including its potential presence in semen, sexual transmission, and its impact on male fertility and semen quality (14).
2. Objectives
The present study primarily aimed to investigate the presence of SARS-CoV-2 RNA in the semen of outpatients with a high viral load in the upper respiratory tract. The secondary aim of the study was to determine any possible implications of SARS-CoV-2 infection and COVID-19 on semen parameters, and the third aim was to illustrate the clinical examination of patients to assess the testicular size and any other abnormalities.
3. Methods
This study included adult male patients who were referred to the Ali ibn Musa Raza Outpatient Service Center affiliated with Babol University of Medical Sciences, Mazandaran, Iran, with laboratory-confirmed SARS-CoV-2 positivity in the upper respiratory tract within July 2020 and February 2021. Ethics committee approval was obtained from the Institutional Review Board at Babol University of Medical Sciences (BMU, Babol, Iran) on May 12, 2020. Participants signed a written informed consent before semen collection.
3.1. Inclusion Criteria
The participants who had a positive one-step real-time reverse transcriptase-polymerase chain reaction (real-time-RT-PCR) assay with the cycle threshold (Ct) value of less than 20 on nasopharyngeal and oropharyngeal samples taken on admission were recruited in this cross-sectional study. Since acute fever or medications used can alter semen parameters and male reproductive function, we enrolled patients who were asymptomatic or had mild symptoms. Other eligibility criteria included age between 20 - 45 years, being sexually active, absence of smoking, alcohol, and drug abuse and addiction.
3.2. Exclusion Criteria
The exclusion criteria included ejaculation problems, varicocele, testicular atrophy and hypogonadism, cryptorchidism, obstructive azoospermia, history of male infertility, using the assisted reproductive technique, scrotal trauma, abnormal genital anatomy, history of scrotal or inguinal surgery, and urogenital or systemic illness diseases or any drugs affecting the semen quality. All participants were required to put on a mask and wash their hands and genitals thoroughly before producing the semen sample. The semen specimen was obtained by masturbation after 2-3 days of abstinence and ejaculated into a sterile, wide-mouthed container. All samples were analyzed by a valid and standard laboratory according to the World Health Organization (WHO) guidelines for semen analysis (17).
All patients underwent a comprehensive genital examination conducted by one expert urologist to assess testicular size, discomfort, and presence of abnormalities, including epididymitis, skin discoloration, redness, swelling, and tenderness. The diagnosis of acute epididimoorchitis was based on the presence of scrotal pain, tenderness, and swelling. Additionally, all suspected cases of epididymitis underwent scrotal ultrasonography to confirm the diagnosis and evaluate the extent of testicular involvement. Among the 63 patients identified, 31 patients were unable to provide a semen sample due to dry ejaculation (1 patient), erectile dysfunction (6 patients), dying prior to enrollment (2 patients), weakness (7 patients), psychological effect of the primary disease (10 patients), or patients who could not meet 2 - 3 days ejaculatory abstinence period before semen sampling (5 patients); therefore, a total of 32 patients were enrolled in the study.
3.3. Viral Nucleic Acid Extraction
Viral RNA was freshly extracted from 300 µL of semen samples using the Ribospin vRD plus Kit (GeneAll, Seoul, South Korea) according to the manufacturer’s instructions. Briefly, for virus dissociation and purification of viral nucleic acid, 500 µL of VL lysis buffer and 5 µL of carrier RNA (1 µg/µL) were added to each sample containing a microcentrifuge tube. The samples were subsequently incubated at room temperature for 10 minutes until being lysed properly. Ribonucleic acid cleanup was performed by a mini spin column (silica matrix) according to the manufacturer’s instructions. Sterile microcentrifuge tubes containing only reaction mixtures were processed simultaneously with the patient’s samples as an extraction negative control.
3.4. Real-Time-Reverse Transcription-Polymerase Chain Reaction for SARS-CoV-2 Detection
After viral RNA extraction, the samples were promptly subjected to one-step real-time RT-PCR analysis. Genome extracted samples were analyzed by LightMix® SarbecoV E-gene kit (TIB Molbiol, Berlin, Germany) with Light Cycler Multiplex RNA Virus Master (Roche), which could detect SARS-CoV-2 (sensitivity = 5.2 copies per reaction). To avoid false negative results, we used exogenous (Equine Arteritis Virus synthetic RNA) and endogenous internal control (Human RNase-P), which monitor the presence of inhibitors in sample extraction and check sampling quality, respectively. One-step real-time RT-PCR was performed using a Step One Plus™ Real-Time PCR System (Applied Biosystems). Each reaction consisted of 5 µL of RNA extract, 4 µL Light Cycler Multiplex RNA Virus Master (Roche), 0.5 µL of primers, and probes in a 20 µL total reaction volume. The synthesis of complementary deoxyribonucleic acid (cDNA) was carried out at 55°C for 3 minutes and instantly followed by the activation of Taq DNA polymerase at 95°C for 30 seconds. A total of 40 cycles were performed, including a denaturation stage at 95°C for 15 seconds and a combined annealing-extension stage at 60°C for 30 seconds. Each real-time RT-PCR run included reaction mixtures without RNA template as a negative control and the SARS-CoV-2 positive controls (LightMix® SarbecoV E-gene kit positive control and a clinical positive control for patients with laboratory-confirmed COVID-19).
4. Results
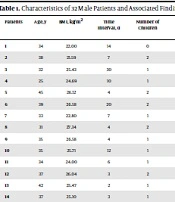
The baseline characteristics and physical information of the 32 participants enrolled in the study are shown in Table 1. Of the 32 participants who provided a semen specimen, 21 (65.6%) patients were in the acute stage of disease (4.67 ± 1.79 days), and 11 (34.4%) patients achieved clinical recovery (13.09 ± 6.76 days). Of the subjects with an acute SARS-CoV-2 infection, 26 cases had no symptoms; nevertheless, 6 patients presented with mild symptoms, such as headache, loss of smell and taste, mild cough, and ague. The participants had a mean age of 36.3 ± 5.28 years old with a mean body mass index (BMI) of 25.46 ± 2.74 kg/m2. All patients were married, and 25 (78.2%) cases had at least one child (Table 1). None of the patients had a recent history of urogenital or hormonal disease. During the physical examination, 28 patients were physically normal with respect to external genitalia and testicular volume; however, acute epididymo-orchitis and testicular tenderness were observed in the genitourinary system of 4 patients in the acute stage of the disease.
| Patients | Age, y | BMI, kg/m2 | Time Interval, day a | Number of Children | SARS-CoV-2 Semen Result | Semen Volume, mL | Sperm Count, 106/mL | Sperm Morphology, % | Total Motility, % | Progressive Motility, % | Leucocytes Detected | Immotile, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | 22.00 | 14 | 0 | Negative | 3.00 | 1 | 2 | 20.00 | 0 | 8 | 100 |
| 2 | 38 | 21.59 | 7 | 2 | Negative | 3.20 | 39 | 3 | 40.00 | 24 | - | 55 |
| 3 | 32 | 25.43 | 30 | 1 | Negative | 5.20 | 88 | 13 | 50.00 | 46 | - | 45 |
| 4 | 25 | 24.69 | 10 | 1 | Negative | 4.80 | 37 | 4 | 40.00 | 38 | - | 45 |
| 5 | 45 | 26.12 | 4 | 2 | Negative | 7.90 | 46 | 3 | 20.00 | 13 | 10 | 70 |
| 6 | 39 | 26.58 | 20 | 2 | Negative | 2.30 | 93 | 12 | 50.00 | 48 | - | 40 |
| 7 | 33 | 22.80 | 7 | 1 | Negative | 4.40 | 26 | 4 | 20.00 | 13 | - | 60 |
| 8 | 31 | 27.34 | 4 | 2 | Negative | 3.00 | 15 | 3 | 40.00 | 5 | 4 | 80 |
| 9 | 35 | 26.58 | 4 | 1 | Negative | 3.00 | 20 | 2 | 30.00 | 20 | - | 65 |
| 10 | 35 | 25.71 | 12 | 1 | Negative | 4.00 | 15 | 5 | 32.00 | 25 | - | 45 |
| 11 | 34 | 24.00 | 6 | 1 | Negative | 3.00 | 12 | 6 | 35.00 | 28 | - | 60 |
| 12 | 37 | 26.04 | 3 | 2 | Negative | 4.80 | 84 | 27 | 51.00 | 41 | - | 45 |
| 13 | 42 | 25.47 | 2 | 1 | Negative | 1.80 | 25 | 3 | 10.00 | 2 | 4 | 95 |
| 14 | 37 | 25.10 | 3 | 1 | Negative | 2.20 | 72 | 36 | 58.00 | 53 | - | 45 |
| 15 | 42 | 26.32 | 4 | 2 | Negative | 4.50 | 14 | 2 | 35.00 | 30 | 4 | 75 |
| 16 | 28 | 30.19 | 8 | 0 | Negative | 5.60 | 16 | 4 | 25.00 | 17 | 12 | 55 |
| 17 | 33 | 31.10 | 7 | 1 | Negative | 4.20 | 6 | 3 | 31.00 | 20 | - | 70 |
| 18 | 40 | 18.00 | 8 | 2 | Negative | 1.50 | 8 | 10 | 28.00 | 15 | 5 | 80 |
| 19 | 37 | 25.39 | 6 | 2 | Negative | 3.50 | 15 | 20 | 38.00 | 25 | - | 45 |
| 20 | 38 | 24.52 | 8 | 0 | Negative | 3.20 | 78 | 18 | 60.00 | 55 | - | 35 |
| 21 | 45 | 27.00 | 7 | 2 | Negative | 2.20 | 28 | 4 | 46.00 | 32 | 6 | 65 |
| 22 | 36 | 23.50 | 4 | 2 | Negative | 5.60 | 36 | 5 | 41.00 | 34 | - | 50 |
| 23 | 30 | 24.30 | 8 | 0 | Negative | 4.80 | 13 | 4 | 32.00 | 21 | 4 | 60 |
| 24 | 37 | 28.00 | 5 | 1 | Negative | 1.20 | 58 | 10 | 50.00 | 40 | 8 | 35 |
| 25 | 38 | 23.20 | 3 | 0 | Negative | 2.80 | 48 | 10 | 47.00 | 36 | - | 50 |
| 26 | 28 | 25.95 | 6 | 0 | Negative | 3.50 | 20 | 28 | 39.00 | 32 | - | 60 |
| 27 | 33 | 25.18 | 11 | 1 | Negative | 2.80 | 45 | 35 | 44.00 | 35 | - | 35 |
| 28 | 45 | 24.39 | 7 | 1 | Negative | 3.60 | 86 | 34 | 60.00 | 50 | - | 35 |
| 29 | 37 | 25.73 | 15 | 1 | Negative | 3.00 | 40 | 15 | 40.00 | 35 | - | 45 |
| 30 | 46 | 33.00 | 5 | 1 | Negative | 4.00 | 10 | 4 | 30.00 | 15 | - | 70 |
| 31 | 33 | 24.76 | 2 | 1 | Negative | 3.00 | 14 | 2 | 39.00 | 28 | 2 | 60 |
| 32 | 30 | 25.00 | 2 | 0 | Negative | 2.50 | 12 | 3 | 35.00 | 10 | 6 | 60 |
Characteristics of 32 Male Patients and Associated Findings of Coronavirus Disease 2019 (COVID-19) Analysis in Semen
4.1. Detection of SARS-CoV-2 RNA in Semen
Endogenous internal control (Human RNase-P gene) was present in the extracted genome of all samples (average Ct of 26.7 ±1.9, range: 23 to 30). In addition, exogenous internal control (Equine Arteritis Virus synthetic RNA), which spiked into each sample, was present in all cases (average Ct of 29 ± 1.7, range: 27 to 33). These results ruled out inhibition during sample extraction, reverse transcription, and PCR amplification and confirmed that all negative results were negative. In total, SARS-CoV-2 RNA was undetectable in all semen samples.
4.2. Semen Characteristics of Patients
Semen specimens of 32 Iranian patients were obtained and assessed for semen parameter values (e.g., semen volume, total sperm count, total and progressive motility, and morphology) (Table 2). The time interval between sample collection and disease onset ranged from 2 days to 30 days (with a median of 6 days). Most of the samples were collected in the acute stage of disease (with a median of 4 days). Semen analysis showed that nine (28.1) patients were diagnosed with oligozoospermia (defined as < 15 million/mL). One case was diagnosed as severe oligozoospermia (defined as less than 5 million/mL). Moreover, 17 (53.1%, 17/32) patients had low sperm motility (defined as < 40% total motility). Furthermore, progressive motility decreased in 18/32 (56.3%) patients (< 32% motile).
| Parameters | Ct Value: 15 - 20 | Ct Value < 15 | P-Value | Effect Size | 95% Confidence Interval of the Difference | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Volume, mL | 3.47 ± 1.01 | 3.77 ± 2.04 | 0.58 | 0.214 | -0.535 | 0.969 |
| Count, 106/mL | 34.83 ± 28.30 | 35.40 ± 26.96 | 0.95 | 0.02 | -0.720 | 0.768 |
| Morphology, % | 12.32 ± 12.23 | 6.30 ± 3.74 | 0.14 | 0.577 | -1.338 | 0.184 |
| Viability, % | 45.61 ± 16.52 | 47 ± 14.18 | 0.82 | 0.08 | -0.66 | 0.845 |
| Total motility, % | 39.40 ± 12.26 | 34.90 ± 11.44 | 0.33 | 0.374 | -1.12 | 0.379 |
| Progressive, % | 28.45 ± 15.73 | 26 ± 12.40 | 0.66 | 0.165 | -0.914 | 0.583 |
| Leucocytes | 5.75 ± 3.10 | 6.75 ± 2.75 | 0.59 | 0.333 | -0.874 | 1.54 |
Semen Parameters of Coronavirus Disease 2019 (COVID-19) Positive Subjects with Cycle Threshold (Ct) Value < 15 and Ct Value 15 - 20
Among the 32 patients, 18 (56.3%) patients had percentages of progressively motile spermatozoa below the WHO reference values (range: 0 - 55%). Additionally, case 1 had complete asthenozoospermia (defined as 100% immotile spermatozoa). Moreover, this study showed a considerable reduction in sperm morphology. Twelve (37.5%) patients had poor sperm morphology, which relates to the recommended criteria of the WHO for diagnosing teratozoospermia (<4% normal). Leukocytospermia was observed in 12 (37.5%) patients. In addition, the present study divided the subjects into two subgroups (as the Ct value < 15 versus Ct ≥ 15 to examine the effect of the high load of a virus on semen parameters. Although it was observed that sperm motility (P = 0.33, Cohen’s d = 0.374) and morphology (P = 0.14, Cohen’s d = 0.577) decreased in both groups, and differences were not statistically significant, a good effect size was observed (Table 2). This is an underpowered study due to the limited size of patients.
Out of the 32 patients, 6 cases had an initial seminal fluid analysis before the COVID-19 attack, which permitted direct comparison in those subjects. The overall semen quality of patients decreased considerably. There was a decrease in total sperm count; total and progressive motility and morphology of all 6 patients were compared before the infection. This study further compared semen parameters from the acute to the recovery phase of the infection. However, no obvious difference in these parameters was observed between the cases either in the acute or recovery stage, probably due to the limited small sample size.
5. Discussion
The present study investigated the presence of SARS-CoV-2 RNA in the semen of asymptomatic and mildly symptomatic Iranian patients with a high viral load in the upper respiratory tract, and genital examination and semen analysis were conducted. There was no SARS-CoV-2 RNA in the semen of patients in the acute or recovery phase of the disease. The real-time RT-PCR assay was highly sensitive (sensitivity = 5.2 copies per reaction based on the kit manual), and this study used exogenous and endogenous internal control to rule out any false-negative result. Several cross-sectional and cohort studies investigated the presence of SARS-CoV-2 RNA in the semen and testicular samples in the acute or recovery phases of infection (8). Based on our knowledge, among these studies, only Li et al. reported the presence of viral genome in 6 patients in the acute (4 of 15 subjects) and recovery (2 of 23 patients) phases of infection. Other study groups did not detect virus RNA in semen samples, which is in agreement with the results of the current study (8, 11-13, 18, 19).
In Li et al.’s study (8), the interval between diagnosis and sample collection was relatively short (2.5 - 7.5 days), compared to other studies. However, the methodology of the real-time RT-PCR assay was not clearly described, potentially leading to false-positive results due to contamination or variations in cutoff values. Moreover, some investigators presume that the presence of a virus in the semen is an outcome of the residual urine shedding, as the genital tract is located in close proximity to the urinary system, and the urethra is a part of both systems (20). Furthermore, the sample collection modality is not described in detail, as in the process of semen collection, if the specimen is not obtained according to aseptic technique, viral particles can be shed in semen from hands or respiratory droplets, giving rise to a false-positive result.
Despite a growing body of research, several limitations have been identified in previous studies examining the presence of SARS-CoV-2 RNA in the male genital system. These limitations include small sample sizes, potential selection bias, lack of comprehensive genital examinations, and a focus on non-severe patients in the recovery stage. Notably, six out of nine studies had sample sizes of less than 17 male subjects, further compromising the statistical power of these investigations. Given the transient nature of viremia and the limited shedding duration (16 - 17 days) in other bodily fluids, these shortcomings might have hindered the accurate assessment of virus prevalence in semen (14). Therefore, if the virus ever existed in semen, it might have been cleared up during the detection time. However, in acute pandemic situations, these studies provided critical information about clinical experiences.
Based on the secondary and tertiary aims of the study, this study investigated the semen parameters and examined the genital tract. Some investigators stated that fever is a common symptom of COVID-19 that can impair scrotal thermoregulation. Fever induced by COVID-19 can alter semen characteristics, such as sperm count and motility, even in the absence of a virus in the semen. It could also have a more deleterious impact among infertile men with altered semen parameters in the basal state (20). Previous studies have suggested that severe clinical signs, fever, and medications could negatively impact semen quality and spermatogenesis. To minimize confounding factors, we focused on recruiting asymptomatic and mildly symptomatic patients with high viral loads and without fever or the need for medication.
The current study revealed impaired semen parameters, including decreased sperm count, motility, and morphology. Additionally, more than 12% of patients experienced scrotal discomfort, epididymo-orchitis, and scrotal wall edema at the time of their SARS-CoV-2 diagnosis. Ultrasound findings included heterogeneous testicular echogenicity, epididymal swelling, and scrotal wall edema. To rule out other potential infections, urine microbiological cultures were performed; however, no infections were detected. Notably, these testicular symptoms were observed predominantly in patients with Ct values less than 15 and mild symptoms, suggesting a possible association with SARS-CoV-2 infection.
The findings of the present study are consistent with those obtained by La Marca et al. and Ediz et al. (21, 22), where they significantly observed higher testicular pain or epididymo-orchitis in severe COVID-19 cases than in the non-severe COVID-19 groups. However, another study (23) on 253 discharged or recovered patients did not demonstrate any scrotal symptoms or orchitis. The difference between the aforementioned results and the results of the current study might be related to the phase of patients’ evaluation (acute or recovery), the load of the virus, hospitalization, and antiviral drugs. About six studies investigated the semen parameters in SARS-CoV-2-positive patients. Ruan et al. studied the semen samples of 74 COVID-19-recovered patients (the mean interval until semen collection: 80 days) and stated that the total semen parameters of recovered patients were higher than the lower reference limit published by the WHO. Whenever compared to the control group, sperm density, total sperm count, and motility meaningfully decreased (13).
Holtmann et al. (24) did not detect SARS-CoV-2 RNA in the semen specimen of acute SARS-CoV-2-positive male subjects (only 2 patients) and recovered patients (18 male subjects). However, they reported impairment of sperm quality (e.g., sperm count and total number of progressive and complete motility) among patients with a moderate infection, compared to mild infection or healthy control group. In this study, recovered patients were stratified based on the severity of the disease and the presence of fever at the time of infection. The fever-positive group had lower sperm concentration and total motility than the fever-negative group. Additionally, lower sperm quality was detected among the recovered patients with moderate disease. In contrast to the findings of the current study and the above-mentioned studies, Guo et al. (25) stated all semen parameters were normal in 23 recovered patients. It should be noted that all the semen specimens came from non-critically ill patients, and they were in the recovery phase (interval 32 days) of infection.
A recent German study showed that asymptomatic and mildly symptomatic COVID-19 patients had higher initial viral loads than hospitalized patients (26). The results of the present study and the above-mentioned studies suggest that COVID-19 might be involved in producing testicular damage and lead to impaired spermatogenesis. This study has certain limitations. Firstly, there was a small sample size. Secondly, the evaluation of SARS-CoV-2 RNA present in seminal fluid during the course of infection via serial sampling could be more informative. Nevertheless, serial semen sampling was difficult in some countries, such as Iran and other Islamic countries. Thirdly, the lack of semen analysis before SARS-CoV-2 infection limited the diagnosis of preexisting male infertility, and only 5 of the studied samples had previous semen analysis for comparison before and after infection. Moreover, there was a lack of appropriate controls. Fourthly, there was no follow-up period. Finally, the preliminary results of this study lack any data about the long-term effects of SARS-CoV-2 on male reproductive function.
5.1. Conclusions
The absence of SARS-CoV-2 RNA in all semen samples suggests a low likelihood of sexual transmission through semen, even during the acute phase of infection. However, the obtained findings raise concerns about possible testicular involvement and an impact on male reproductive function. Further research is warranted to elucidate the mechanisms underlying these effects and to determine their reversibility. Additionally, clinicians should remain vigilant and carefully evaluate patients with genital symptoms regardless of their systemic presentation, given the potential for atypical manifestations of SARS-CoV-2 infection.