1. Background
Streptococcus pneumoniae is a major causative agent of morbidity and mortality worldwide (1). The world health organization (WHO) estimates that annually 1.6 million people die of pneumococcal disease (2). The introduction of conjugated vaccines has declined the number of deaths due to pneumococcal infections, especially among children. However, pneumococcal infections remain an important disease among children under 2 years-old, immune compromised people, and the elderly aged above 65 years (3).
Accurate identification of S. pneumoniaecan track the organ-based infection. A robust method for detection of S. pneumoniae can decrease the morbidity and mortality rate of pneumococcal infections, especially in developing countries. Therefore, a reliable method is essential to accurately treat patients and control pneumococcal disease (3, 4).
Diagnosis of pneumococcal infection includes conventional culture-based and molecular methods. The culture-based methods include optochin susceptibility, agglutination, and bile solubility, which are routinely used for differentiation of S. pneumoniae from other species such as S. viridans. However, these assays can lead to misidentification of this agent due to the emergence of optochin resistant pneumococcus (5). Furthermore, antibiotic therapy before sample collection or activation of autolysins during sample transmission can lead to false negative results (5, 6).
Latex agglutination, counter immunoelectrophoresis, and immunochromatography have low sensitivity for detection of S. pneumoniae (7). Recently, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) proposed for rapid diagnosis of mitis group streptococci sometimes has produced inaccurate results (8). Therefore, molecular methods have been developed to accurately diagnose S. pneumoniae in isolates and clinical samples.
Molecular methods have higher sensitivity for the detection of S. pneumoniae among patients under antibiotics therapy (9). They are used for diagnosis of pneumococcal infections based on identical genes i.e.: pneumolysin gene (ply), autolysin gene (lytA), and spn9802. Differentiation of S. pneumoniae is not reliable by detection of these genes in the genus (8). Thus, it is essential to use species-specific genes for accurate diagnosis of S. pneumoniae from clinical samples.
2. Objectives
In the present study, we aimed to evaluate the efficacy of lytB gene along with lytA gene for detection of S. pneumoniae in isolates and clinical samples using conventional and real-time PCR methods.
3. Methods
3.1. Clinical Isolates
In this cross-sectional study, a total of 560 clinical specimens were collected from patients with suspected pneumococcal infections in teaching hospitals of Tehran during February-September 2015. The specimens were cerebrospinal fluid, ascites fluid, trachea aspirates, blood, pleural fluid, sputum, bronchoalveolar lavage, ear discharge, synovial fluid, and eye discharge. The mentioned samples were cultured on 5% sheep blood agar (Merck, Germany) and incubated in 5% CO2 at 37°C for 24 hours. Confirmation of suspected S. pneumoniae colonies was performed by gram staining, bile solubility, and optochin (MAST, UK) susceptibility tests. All isolates were cultured in trypticase soy broth (Merck, Germany) containing 10% glycerol (Merck, Germany) and stored in -70°C (10). This study was approved by the ethics committee of Tehran University of Medical Sciences (code: 26772).
3.2. Antibiotic Susceptibility Testing
The antibiotic susceptibility test was performed using Kirby-Bauer disk diffusion method on Mueller Hinton agar (Merck, Germany) containing 5% sheep blood for the following antibiotics: vancomycin (30 μg), tetracycline (30 μg), clindamycin (2 μg), cotrimoxazole (25 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), and erythromycin (15 μg) (MAST, UK). Following the 24-hour incubation at 37°C, the inhibition zones were measured according to the guidelines of clinical and laboratory standards institute (CLSI) and reported as resistant (R), intermediate (I), and susceptible (S) (11). Antibiotic susceptibility test for penicillin and cefotaxime was performed using minimum inhibitory concentration (MIC) determination by serial microdilutions method according to CLSI guidelines (11). Streptococcus pneumoniae ATCC49619 were used for quality control. All statistical analyses were performed using SPSS, version 22.
3.3. DNA Extraction
DNA of the isolates and clinical samples was extracted by DNA extraction kit according to the manufacturer’s instructions (DNeasy Blood and Tissue Qiagen, Germany). The extracted DNA was stored at -20°C.
3.4. PCR Assay
46 isolates were examined to evaluate the presence of lytA and lytB genes with primers depicted in Table 1 (12). Final volume of reaction was 25 µL hot start Taq master mix kit (Sinaclon, Iran) that included: 12.5 μL of 2x hot start Taq master mix (containing 3 mM MgCl2, 0.4 mM of each dNTP, 0.08 U/µL Taq DNA polymerase), 2 µL DNA, 1 uL of each primer (10 pmol), and 11.5 µL ddH2O. Initial step was performed at 94°C (5 minutes), 35 amplification cycles of 94°C (30 seconds), 58°C for both the genes (35 sec), 72°C (1 minute), and final extension at 72°C (10 minutes). The PCR products were electrophoresed on 1% agarose gel (Biotium Inc, USA).
| Primers | Sequences | Products Size, bp | Ref. |
|---|---|---|---|
| lytA | F:5′-ACGCAATCTAGCAGATGAAGCA-3′ | 319 | (12) |
| R:5′-TCGTGCGTTTTAATTCCAGCT-3′ | |||
| lytB | F:5′-ACAGAGGAAGAAGGTTGATGAAG-3′ | 150 | This study |
| R: 5′-ACTCATTCGCTGCTTGACTG -3′ |
3.5. Real-Time PCR Assay
The real-time PCR assays were performed using TaqMan Universal PCR master mix on isolates, 46 culture-negative specimens (46/514) and 46 culture-positive specimens. Culture-negative samples were randomly selected from 514 specimens. The real-time lytB and lytA PCR assays were performed in a final volume of 20 µL by hot start HiFi real-time PCR master mix consisting of 4 µL hot start master mix, 1 µL each primer (10 pmol) ( Table 2) (13), 1 µL probe (10 pmol), 2 µL DNA and 11 µL ddH2O. Streptococcus pneumoniae ATCC49619 was used as the positive control. The applied amplification program is listed in Table 3.
| Primers and Probes | Sequences | Ref. |
|---|---|---|
| lytA primers | F:5′-ACGCAATCTAGCAGATGAAGCA-3′ | (13) |
| R:5′-TCGTGCGTTTTAATTCCAGCT-3′ | ||
| lytA probe | 5′-FAM-TGCCGAAAACGCTTGATACAGGGAG-3-′BHQ1 | (13) |
| lytB primers | F:5′-ACAGAGGAAGAAGGTTGATGAAG-3′ | This study |
| R: 5′-ACTCATTCGCTGCTTGACTG -3′ | ||
| lytB probe | 5′-FAM-AGCTAGTCCAGAGGGTGCAATGGCT-3′-BHQ1 | This study |
| Step | Temperature, °C | Time | Cycle. No, X |
|---|---|---|---|
| Initial Denaturing | 95 | 10 Minutes | 1 |
| Denaturing | 95 | Seconds 15 | 40 |
| Annealing + Extension | 60 | Seconds 60 | 40 |
4. Results
4.1. Bacterial Isolates
A total of 46 isolates of S. pneumoniae were isolated from 560 clinical specimens by biochemical assays. All the 46 isolates were positive in bile solubility test and all of them except one isolate were susceptible to optochin. The optochin resistant isolates were confirmed as S. pneumoniae by both conventional and real time PCR methods.
4.2. Antibiotic Susceptibility Test
52% (24/46) of the isolates were non-susceptible to at least 3 antibiotics and exhibited multiple drug resistance (MDR) (14). The isolates with resistance to cotrimoxazole, erythromycin, and tetracycline were the most cases of MDR. Antibiotic resistance profile of the isolates is shown in Table 4. Isolates causing non-meningitis infections were resistant to penicillin (MIC ≥ 8 µg/mL). Moreover, 3 of the non-meningitis isolates were intermediate resistant pneumococci (MIC = 4 µg/mL). Overall, 10 (23.8%) non-meningitis isolates were penicillin-non-susceptible pneumococci (PNSP). Moreover, 2 out of 4 meningitis isolates with penicillin MICs of 0.12 µg/mL and 0.5 µg/mL were penicillin-resistant according to CLSI guidelines (11). 10 non-meningitis isolates were cefotaxime-resistant (MIC ≥ 4 µg/mL), 2 were intermediate resistant (MIC = 2 µg/mL), and 2 meningitis isolates were cefotaxime-resistant (MI ≥ 2 µg/mL).
| Antibiotics | Number of Resistant Isolates (%) |
|---|---|
| Vancomycin | 0 |
| Ciprofloxacin | 6 (13.04) |
| Penicillin (non-meningitis) | 7 (16.7) |
| Chloramphenicol | 8 (17.39) |
| Cefotaxime (non-meningitis) | 10 (23.8) |
| Clindamycin | 19 (41.3) |
| Tetracycline | 24 (52.17) |
| Erythromycin | 25 (54.34) |
| Cotrimoxazole | 36 (78.26) |
4.3. Molecular Assays for Detection of S. pneumoniae
All the isolates, which had been identified biochemically, were confirmed by the conventional and real-time PCR of lytA and lytB genes. Moreover, one isolate was optochin-negative, but molecular assay of both genes confirmed it as S. pneumoniae. Totally, 46 isolates were identified as pneumococci by real-time lytB and lytA PCR assays. PCR assays were performed on the isolates and the obtained results were concordant with those of real-time PCR.
Since the real-time lytB and lytA PCR assays performed well on the isolates, we evaluated the diagnostic performance of the primers for the detection of S. pneumoniae in clinical specimens. The real-time PCR was done on 46 culture-positive and 46 culture-negative specimens. All the 46 culture-positive samples were positive in both real-time PCR assays with low CT values. Therefore, this is indicative of the presence of S. pneumoniae in the specimens. The median CT values of the lytB and lytA PCR assays were 23.6 (CT range 18.6 to 30.2) and 22.8 (CT range 18.1 to 29.8), respectively.
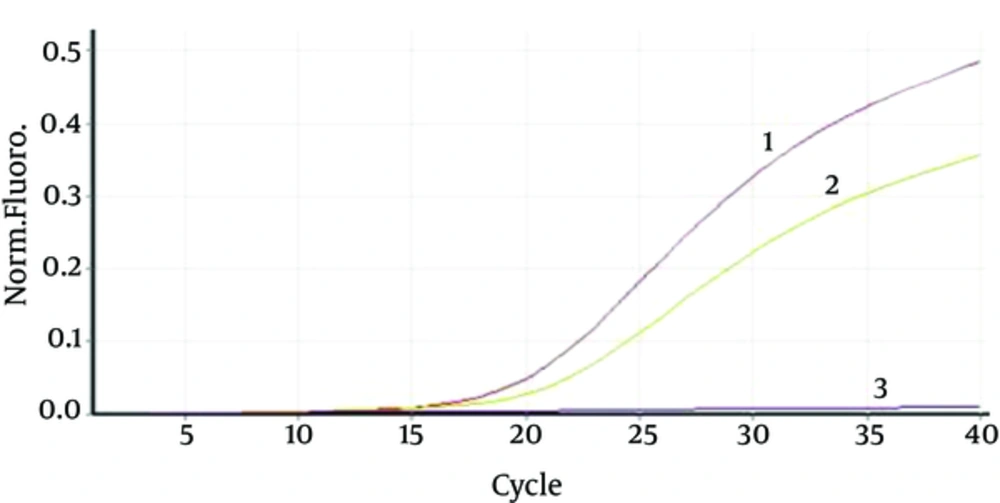
Forty six culture-negative samples were randomly selected for real-time PCR assays including: trachea (n = 4), blood (n = 12), plural fluid (n = 6), sputum (n = 15), and pulmonary lavage (n = 9). Among 46 culture-negative specimens, one sample (sputum) was positive for both the genes. The sample belonged to a patient who received antibiotic. CT values of the real-time lytB and lytA assays for this sample were 33.6 and 32.8, respectively (Figure 1).
5. Discussion
In this study, all biochemically identified S. pneumoniae isolates were confirmed by conventional and real-time PCR assays of lytA and lytB genes. One of the isolates was resistant to optochin, confirmed as S. pneumoniae by molecular assays. Use of conventional methods for diagnosis of S. pneumoniae has limitations due to emergence of optochin resistant pneumococci, time-consuming, and false negative results (6). In previous studies, it has been shown that sensitivity of lytA real-time PCR assay in the diagnosis of S. pneumoniae isolates is valid (7, 8, 13). In our study, all the isolates identified in biochemical assays were also confirmed by PCR and real-time PCR assays. Thus, the sensitivity of the real-time lytB assay was similar to that of real-time lytA assay for the diagnosis of S. pneumoniae isolates.
Close species of pneumococci such as S. pseudopneumoniae, S. mitis, and S. oralis can colonize in nasopharynx. Transmission of genetic elements between pneumococci and close species can occur, leading to misidentification of these species (15). Commonly used genes for the diagnosis of S. pneumoniae such as lytA, ply and spn9802 exist in other members of the viridans group streptococci, affecting the specificity of the molecular assays (16). Furthermore, low levels of bacteria can cause false-negative results in direct detection of clinical samples. This problem can be solved using sensitive molecular assays such as real-time PCR and improved methods of DNA purification (17).
During recent years, the presence of lytA gene has been proposed for diagnosis of S. pneumoniae. The strategy of real-time PCR targeting the lytA gene has been advised by WHO for detection of pneumococcal DNA in clinical samples (13, 15). However, lytA gene has homology among pneumococci and non-pneumococci isolates, which can lead to misidentification of S. pneumoniae. The homology is due to point mutations and deletion mutations in the lytA gene (15). Previously, two copies of lytA gene were characterized in the genome of S. pseudopneumoniae isolated from clinical samples (17).
Altogether, the specificity of lytA real-time PCR for the diagnosis of S. pneumoniae is doubtful and despite the common target gene, there have been various results. Several studies have shown that this method is specific, but some others have not confirmed these findings (13, 18). It has been shown that this method cannot differentiate S. pneumoniae from S. pseudopneumoniae isolates. For the differentiation of these two species, the spn9802 gene has been proposed. Although this gene can differentiate S. pneumoniae from S. pseudopneumoniae, in direct examination from clinical samples it is not applicable (8).
Currently, lytA real-time PCR assay is an interesting strategy for direct detection of S. pneumoniae in clinical samples that needs the association with a second gene (19). In the present study, the real-time PCR assay was done on 46 culture-negative and 46 culture-positive specimens. All the culture-positive samples (46/46) were positive for these genes and one of the culture-negative samples (1/46) was positive for both genes. None of these two genes were observed in the remaining 45 samples. Overall, the both genes were similar for detection of S. pneumoniae in isolates and clinical samples. More studies need to perform on streptococcal strains by lytB gene in order to more fully evaluate the specificity of this gene.
In this study, 23.8% of non-meningitis isolates were penicillin non-susceptible, which is similar to studies from other parts of the world. Penicillin non-susceptible isolates in Turkey, North America, and Europe were 19.1%, 9% - 24%, and 0% - 43%, respectively (20). In previous studies from Iran, the antibiotic susceptibility profile has been different and it has been totally shown that resistance to erythromycin, cefotaxime, cotrimoxazole, chloramphenicol, and penicillin is increasing (21, 22). In the present study, most of MDR cases were resistant to cotrimoxazol, erythromycin, and tetracycline. Because of high resistance to penicillin and more spread of MDR strains, misidentification of pneumococci leads to the increase in false report of non-susceptible isolates (15).
5.1. Conclusions
The lytB is similar to lytA in sensitivity for diagnosis of S. pneumoniae in isolates and clinical samples based on both molecular methods. The results confirmed the applicability of real time PCR based on lytB genes for detection of S. pneumoniae.