1. Introduction
Psittacosis is an infectious disease caused by Chlamydophila psittaci. According to statistics, the ratio of C. psittaci pneumonia to community-acquired pneumonia (CAP) ranged from 0.5% to 15%, with an average of 1% (1). However, in severe cases, the incidence of C. psittaci is as high as 8% (2). The clinical manifestations of psittacosis vary greatly, usually involving the respiratory system, characterized by high fever, dry cough, pulse temperature dissociation (fever without increased pulse rate), with a high proportion of extrapulmonary manifestations, such as neurological symptoms (headache and delirium) and digestive tract symptoms (abdominal pain and vomit) (3). Due to the high technical difficulty of chlamydia culture, samples might be infected during the procedure. Polymerase chain reaction (PCR) and microimmunofluorescence (MIF) tests can be used as evidence for the diagnosis; nevertheless, false negative results can be caused by many infectious factors. None of the above-mentioned detection methods are popular in Chinese hospitals and are only performed in specialized facilities.
As an emerging technology for pathogen detection, metagenomic next-generation sequencing (mNGS) demonstrates notable sensitivity and informativeness. This capability encompasses the identification of established pathogens and the discovery of novel entities, thereby enhancing the diagnostic yield for pathogens and providing guidance for the judicious use of anti-infective pharmaceuticals. Herein, we present a case of C. psittaci causing severe pneumonia, bloodstream infection, and cerebrovasculitis with etiologic diagnosis aided by mNGS and clinical analysis, meanwhile accompanied by pulmonary embolism (PE). The etiological diagnosis was facilitated by the integration of mNGS and clinical analysis, thereby highlighting the diagnostic potential of this advanced pathogen detection technology.
2. Case Presentation
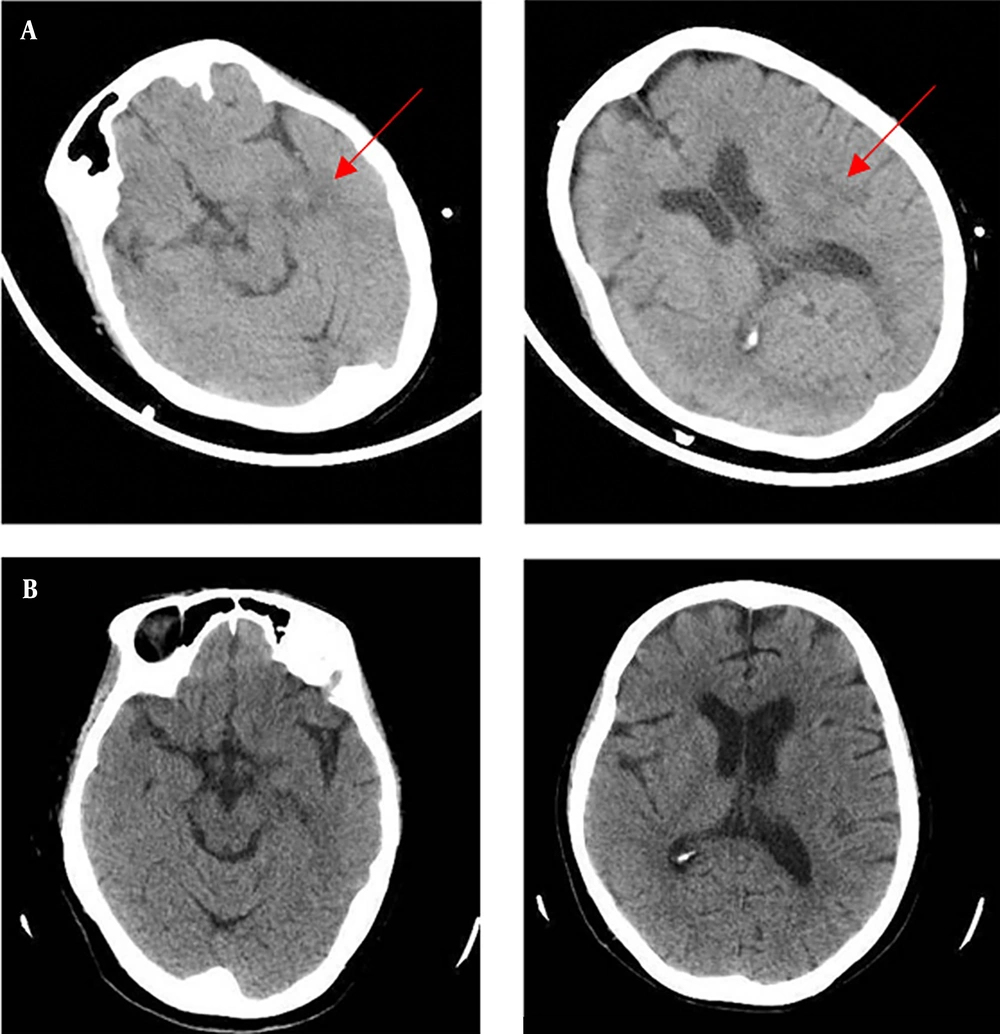
A 72-year-old woman was admitted to our emergency department of Zhejiang Hospital due to a 5-day history of fever and a 7-hour delirium, accompanied by progressive dyspnea, on November 22, 2021. She presented with a high fever 5 days before admission. The peak temperature was 40°C, with dry cough and mild headache. She was observed to have stumbled 2 days ago (not fallen down) and was sent to the local hospital by family members. Laboratory data in the local hospital revealed a white blood cell (WBC) count of 3.92 × 109/L with an elevated neutrophil ratio of 93.9%. The concentrations of C-reactive protein (CRP) and procalcitonin (PCT) were 308.9 mg/L and 3.585 ng/mL, respectively. Erythrocyte sedimentation rate (ESR) was 73 mm/h. A head computed tomography (CT) scan showed multiple nodules with peripheral edema in the left cerebral hemisphere (November 20, 2021, Figure 1A). A chest CT scan indicated an extensive inflammatory lesion with partial consolidation in the left upper lung (November 20, 2021, Figure 2A). After the poor response to the therapy of cefoperazone/sulbactam, antipyretic and fluid infusion, she came to our emergency.
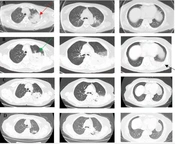
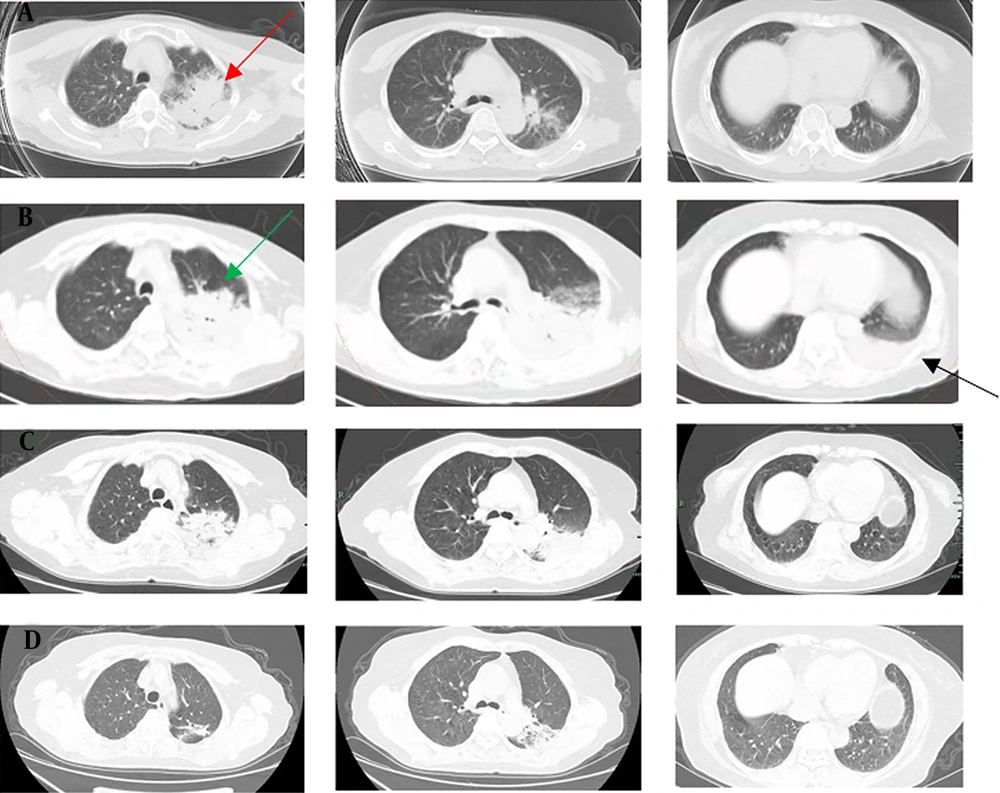
A, chest CT scan (November 20, 2021) indicated extensive inflammatory lesion with partial consolidation (red arrow) in the upper left lung; B, Enhanced pulmonary artery CT scan (November 24, 2021) indicated a local suspicious embolism in the right lower pulmonary artery, and consolidation progressed significantly in the left lung (green arrow) with left pleural effusion (black arrow); C, CT re-examination (November 30, 2021) showed that the lesions were obviously absorbed and pleural effusion disappeared; D, CT re-examination (December 25, 2021) showed further improvement.
Head magnetic resonance imaging (MRI) was not available during staying in the emergency department due to dysphoria. Arterial blood gas analysis showed a pH of 7.473, PaO2 of 85.6 mmHg, PaCO2 of 23.2 mmHg, lactate of 1.3 mmol/L, and oxygenation index of 171.2. White blood cell count was 5.3 × 109/L with a neutrophil ratio of 95.7%. The concentrations of CRP and PCT were 257.7 mg/L and 2.9 ng/mL, respectively. The erythrocyte sedimentation rate was 73 mm/h. D- dimer was 4090 ng/mL (normal range: 0 - 500). The albumin level was 27.5 g/L. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 60U/L and 123U/L, respectively. Lactate dehydrogenase (LDH) was 336 U/L (50 - 240). Creatine kinase (CK) and creatine kinase isoenzyme (CK-MB) were in the normal range. She has no history of chronic diseases and does not smoke or use illicit drugs; however, she reported that she keeps four chickens at home, a possible source of C. psittaci infection. She is a housewife and does occasional farm work. She resides in Zhejiang province, eastern China.
When she was admitted to our department (day 1) for further treatment, her vital signs were as follows: Body temperature 38.0°C, pulse rate 115 beats/min, respiratory rate 25 breaths/min, blood pressure 125/80 mmHg, and pulse oxygen saturation 95% with a fraction of inspired oxygen (FiO2) of 0.5. She was in a state of delirium. Moist rales could be heard in the left upper lung, and she presented with mild cyanosis. No audible murmur on cardiac auscultation was detected. Tenderness and hepatosplenomegaly were not detected, and neurological tests were negative. White blood cell count was 4.8 - 3.8 × 109/L with a neutrophil ratio of 93.2 - 90.3%. C-reactive protein and PCT were 268 - 217.1 mg/L and 1.59 - 2.9 ng/mL, respectively. The albumin level was 23.6 - 24.6g/L. Aspartate aminotransferase and ALT were 56 - 60 and 77 - 112 U/L, respectively. Lactate dehydrogenase was 320 - 396 U/L. Creatine kinase and CKMB were still normal. D-dimer was 17690 - 21790 ng/mL. Other laboratory tests of plasma were negative, such as respiratory viruses, human immunodeficiency virus (HIV) and syphilis test, galactomannan antigen test, cryptococcal antigen agglutination test, and viral hepatitis. All indicators for autoimmune diseases were negative. The sputum and blood cultures for both fungi and bacteria and the sputum acid-fast bacillus test were negative.
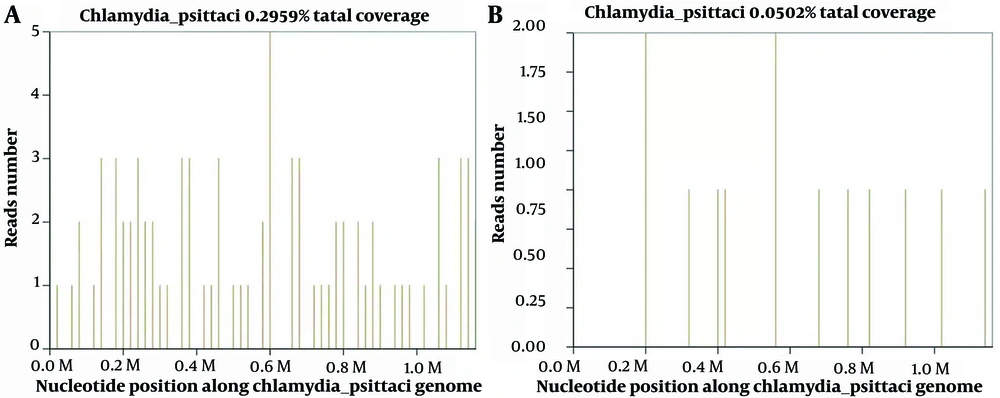
After admission, the patient was given empirical antimicrobial therapy of piperacillin/tazobactam (4.5 g, every 8 hours, intravenous drip) and azithromycin (0.5 g per day, intravenous drip), together with supportive treatment. Lumbar puncture showed an intracranial pressure of 120 mmH2O, and cerebrospinal fluid (CSF) was transparent and colorless. Cerebrospinal fluid cytology and biochemistry showed that WBC counts 7 × 106/L, red blood cell count 18 × 106/L, protein 0.391 g/L (0.15 - 0.4), glucose 3.48 mmol/L (2.5-3.9), chloride ions 124 mmol/L (121.0 - 129.0), and adenosine deaminase (ADA) 5 U/L (< 40). Pandy’s test was negative. In pursuit of elucidating the etiological underpinnings, we dispatched the patient's blood and CSF specimens for mNGS analysis on November 23, 2023. Ink stain and cryptococcal antigen in CSF were both negative. The mNGS of the plasma identified 33 sequence reads corresponding to C. psittaci, together with 16 sequence reads of Human gammaherpesvirus 4, and the mNGS of the CSF identified 11 sequence reads corresponding to C. psittaci, without any other pathogens (Figure 3).
The patient was diagnosed with a severe C. psittaci infection, both pulmonary and extrapulmonary, including bloodstream infection and central nervous system infections. Therefore, azithromycin was switched to doxycycline on day 2 (100 mg, every 12 hours, oral). Piperacillin/tazobactam continued for prophylaxis therapy in spite of the negative results of gram-negative bacteria. Body temperature returned to normal, dyspnea was relieved, and consciousness went back to normal after 48 hours of treatment. In accordance with temperature, the neutrophil predominance dropped continuously, along with CRP, PCT, and ESR. The head CT scan showed no findings of acute infection or cerebral hemorrhage on day 5 (Figure 1B).
Since the onset, the D-dimer remained at a high level, and relevant examinations were arranged. Enhanced pulmonary artery CT scan on day 3 indicated a local suspicious embolism in the right lower pulmonary artery, and consolidation progressed significantly in the left lung with left pleural effusion (Figure 2B). Therefore, low molecular weight heparin (LMWH) (4000 IU, every 12 hours, subcutaneous injection) was administrated. No other arteriovenous thrombosis was observed in the blood vessels, and there was no abnormality in cardiac ultrasound. Chest CT reexamination on day 9 showed that the lesions were absorbed, and the pleural effusion disappeared (Figure 2C). The symptoms of shortness of breath and cough were alleviated, and she was discharged on the 11th day without obvious complaint. The patient continued to receive treatment (doxycycline, 100 mg, every 12 hours, for 10 more days; rivaroxaban, 20 mg per day, at least 3 months). There was a further improvement in imaging (Figure 2D), and the case is being followed up on at present.
3. Discussion
Psittacosis is a zoonosis caused by C. psittaci, a gram-negative intracellular bacterium. Most cases are those who have close contact with birds or poultry, such as farmers raising and selling poultry, veterinarians, and domestic ornamental birds (4). About 40% of the patients have no clear history of exposure to poultry but had exposure to poultry excreta or feather products; therefore, we emphasize the importance of a definite history in making the correct diagnosis (5, 6). Autumn and winter can be the peak seasons for psittacosis, with a study from Australia showing that 69% of cases occurred during the cold months (March to May in the southern hemisphere) (4). Most infected individuals are adults aged 45 - 70 years; nevertheless, this patient was older. There is no significant difference in gender. However, parrots were the most common source of infection in women and pigeons in men (7).
Psittacosis is mainly spread by inhalation through the respiratory tract with secretory particles containing chlamydia. Chlamydophila psittaci first goes into the reticular epithelial cells of the liver and spleen to proliferate, pass through the blood flow, and reenter the lungs and other organs. Therefore, psittacosis is a systemic infection disease mainly referred to the respiratory system. The chlamydial developmental cycle in parrots exhibits a distinctive biphasic developmental morphology, comprising an infectious yet metabolically quiescent elementary body (EB) and a non-infectious, metabolically active reticulate body (RB). It is noteworthy that, throughout the replication cycle, interconversion between EB and RB is feasible (8, 9).
In this study, the central nervous system manifestations in the patient are posited to represent a vascular inflammation underpinned by cell-mediated immunity incited by the parasitism of Chlamydia within the cerebral blood vessels. After antibiotic intervention, a consequential impediment in the transition of Chlamydia from its EB to reticulate bodies RB is observed, thereby arresting their inherent intracellular maturation. Consequently, a subsequent head CT scan reveals the prompt resorption of the lesions. In addition, she had a special PE complication. Several cases of C. psittaci infection complicated with lower limb arterial occlusion with or without cardiac involvement have been reported earlier (10); however, similar reports are rare. Whether PE is associated with psittacosis infection remains to be determined.
Although it is difficult to distinguish psittacosis pneumonia from those of other types of CAP by clinical manifestations and routine laboratory tests, they still show specificity for atypical pneumonia in radiology. Chest CT scans usually show different degrees of exudation and consolidation. Most of them are unilateral and single lobe consolidation; the second is unilateral multilobed consolidation and bilateral multilobed consolidation. The extent of consolidation is closely related to the severity of the infection. Patients with multilobed consolidation have a higher risk of respiratory failure. Consolidation is mainly in the lower lobe. Hilar lymph node enlargement or pleural effusion is rare. However, it is still difficult to distinguish it from Legionella pneumonia based on the above-mentioned characteristics. In this case, chest CT showed extensive multilobar pneumonia with pleural effusion but not bilateral, which was by severe cases but no need for ventilation, partly due to the timely diagnosis and treatment.
In recent years, with the popularization of mNGS, the number of reported C. psittaci infections has been increasing, especially in severe cases, which supports considerable timely diagnosis and treatment (11, 12). Metagenomic next-generation sequencing constitutes a sequencing methodology facilitating comprehensive analysis of the microbiome within clinical specimens. This approach involves the extraction of nucleic acids from the entirety of microorganisms present, employing genomics to scrutinize the deoxyribonucleic acid (DNA) composition of the complete microbial spectrum and comprehend community functionality. The distinctive capability of mNGS to promptly and precisely identify potential pathogens not predefined in established categories confers notable advantages in the diagnosis of infrequently encountered pathogens (13).
Teng et al. (14) compared serological tests and PCR detection for 5 cases confirmed by mNGS, which showed that only 2 cases were positive for antibodies; however, all cases were negative for PCR, indicating that mNGS might be more sensitive than antibodies and PCR in diagnosis. In this patient, multiple mNGS tests were performed, including plasma and CSF, and C. psittaci sequences were detected in both samples. Plasma mNGS detected C. psittaci together with 16 sequence reads of Human gammaherpesvirus 4; nevertheless, the chest images did not support the features of viral pneumonia, and the body temperature dropped to normal without any subsequent antiviral treatment, which in turn did not support the diagnosis of viral infection. There was no sequence read corresponding to other probable pathogens in the CSF. The mNGS of aseptic blood and CSF specimens, combined with the chest CT scans, help to make an etiologic diagnosis. We finally make the diagnosis of C. psittaci pneumonia, bloodstream infection, and cerebrovasculitis. Due to the high risk of bronchoscopy and difficulty in taking respiratory tract specimens, no bronchoalveolar lavage fluid (BALF) samples were obtained.
Tetracycline antibiotics are the first choice for the treatment of C. psittaci infection, including tetracycline, doxycycline, and minocycline. In severe cases, doxycycline can be administered intravenously, and the temperature usually returns to normal within 48 hours. In China, doxycycline and azithromycin are rarely used as the first choice for severe pneumonia; nevertheless, fluoroquinolones are commonly used or in combination for severe pneumonia. Most patients recover well after receiving fluoroquinolones (5, 14). There are also cases of patients who do not improve after fluoroquinolone treatment but get better with combination or change to doxycycline (4). The course of medication should last 14 - 21 days; otherwise, insufficient treatment might easily lead to relapse (15). Fortunately, the patient recovered quickly, benefiting from targeted treatment. To find the optimal treatment, further case-control studies with more samples are needed in the future.
3.1. Conclusions
In conclusion, with the extensive application of mNGS in difficult, rare, and critical cases, C. psittaci infection has attracted increasing attention. Clinically, psittacosis should be taken into consideration in patients with high fever accompanied by large consolidation of the lungs, with a headache or mental disorders, or with high myoenzymes, especially in cases with severe infections but lack of response to empirical antibacterial therapy. Epidemiological history should be taken in detail, and the mNGS tests should be performed timely to identify the pathogen, transfer it to the targeted treatment as early as possible, shorten the progress of the disease, and improve the prognosis.