1. Background
Hematological malignancies are among the common cancers in Iran. A 2014 study using pathology and cytology data from 60 medical universities across the country found that leukemia was the sixth most common cancer among men and fifth among women (1). The Global Cancer Observatory estimates that by 2020, Iran will see over 131,000 new cancer cases, with more than 12,000 due to the three common hematological malignancies: Leukemia, lymphoma, and multiple myeloma. These malignancies rank third in incidence after breast and gastric cancers and are the third leading cause of cancer-related death, with a mortality rate of 8,000 per year following gastric and lung cancers (2).
Invasive fungal infection (IFI) is prevalent among immunocompromised individuals, particularly those with hematological malignancies, and carries a high mortality rate. A population-based study in France from 2001 to 2010 identified about 36,000 IFI cases, estimating an incidence rate of 5.9 per 100,000 in the general population and 4% among patients with hematological malignancies. The mortality rate was 27.6%, with the most common fungi being Candida spp. (43%), Pneumocystis jirovecii (26%), and Aspergillus spp. (24%) (3). In Italy, a study of approximately 12,000 patients with hematological malignancies showed that 4.6% developed IFI, with a mortality rate of 39%.
The most affected group was patients with acute myeloid leukemia (AML), primarily challenged by Aspergillus and Candida spp. (4). A meta-analysis of about 17,000 patients revealed a 6.3% incidence of invasive Aspergillosis (IA) (5). Additionally, a 20-year autopsy study in the United States from 1998 to 2008 indicated a decrease in the incidence rate of IA from 0.12 to 0.07 in patients with hematological malignancies. Although the prevalence of IFI in these patients has decreased, the incidence of visceral candidiasis has increased (6).
2. Objectives
Considering the prevalence of hematological malignancies in Iran and the limited data on fungal infections in these patients, our study aims to investigate the frequency, risk factors, and mortality rate of IFI in hospitalized patients with hematological malignancies.
3. Methods
3.1. Study Design
We conducted a retrospective cross-sectional study using medical registry data from April 2020 to September 2021 at two university hospitals, Imam Reza and Khanevadeh, in Tehran, Iran.
3.2. Patient Selection and Study Protocol
All registries of hospitalized patients with hematological malignancies were reviewed and selected those who had received antimicrobial treatment for more than four days, with blood, sputum, and urine cultures revealing no bacteria responsible for the infection. Patients admitted for chemotherapy, blood product transfusion, infectious diseases lasting three days or less, or those admitted to non-hematological wards were excluded. Demographic characteristics, history of diabetes, corticosteroid use, type of hematological malignancy, and the number of chemotherapy treatment courses were collected.
The IFI defined as either a proven or probable diagnosis, according to the criteria of the European Organization for Research and Treatment of Cancer and the Mycoses study group Education and Research Consortium (EORTC/MSGERC) (7). Proven cases required a positive blood culture or PCR for fungi and the presence of yeast cells in histopathologic or direct microscopic examination. Probable cases were defined by a positive bronchoalveolar lavage (BAL) culture or PCR for fungi, recent history of neutropenia, presence of at least one typical finding on pulmonary computed tomography (CT), such as dense well-circumscribed lesions, air crescent sign, cavity, and consolidation, and mycological evidence including any mold recovered by sputum or BAL culture and galactomannan antigen.
3.3. Microbiological Method
All patients underwent mycological assays using blood, urine, and sputum cultures on blood agar media. In both hospitals, 5 mL of peripheral blood was inoculated into a bottle and incubated in the BACTEC system. The first report was available after 72 hours, and the second report after 15 days of incubation. Additionally, serum galactomannan levels and PCR for mycosis from sputum or BAL were conducted on patients exhibiting pulmonary symptoms or signs on CT scans.
3.4. Statistical Analysis
SPSS-26 software was employed by IBM Corporation to analyze the data. The Kolmogorov-Smirnov test assessed the normality of the data. Furthermore, the Mann-Whitney U test was used to compare quantitative data, while chi-square and Fisher exact tests were utilized for qualitative data, with significance set at the 95% level.
4. Results
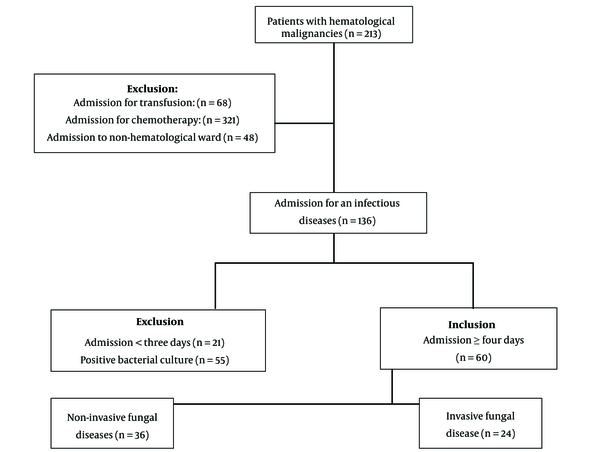
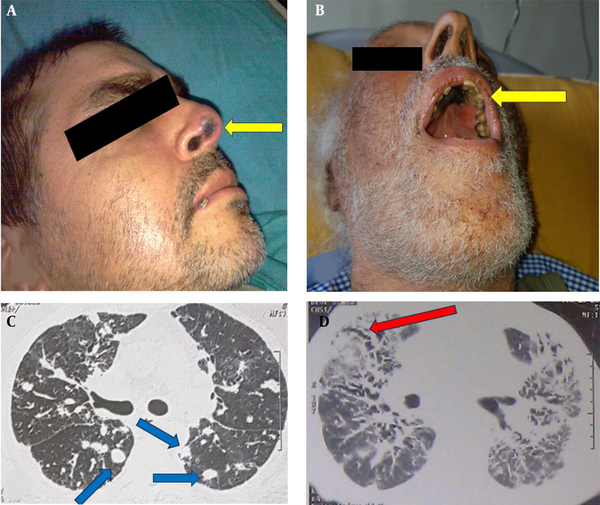
The registries of 213 patients with 573 hospitalization episodes were reviewed and selected 60 patients hospitalized for at least four days for antimicrobial treatment (Figure 1). The mean age was 57.1 ± 17, with an age range of 20 - 86, and a male-to-female ratio of 38/22. None of the patients received antifungal prophylaxis. The most common hematological malignancy was AML, found in 21 patients (35%), and the most prevalent symptom upon admission was fever, noted in 38 patients (63.3%). Table 1 shows the demographic characteristics and primary symptoms of the patients with hematological malignancies and IFI. Table 2 compares these variables in the patients with hematological malignancies, with and without IFI. We identified 24 out of 60 patients (40%) diagnosed with IFI, which represents 11.3% of total patients—these included three proven (5%) and 21 probable (35%) cases. The proven diagnoses comprised three cases of Candida spp. in blood cultures. Probable cases included three Aspergillus spp. in BAL cultures, one PCR-confirmed Aspergillus spp. in a BAL sample, nine cases with multiple pulmonary nodules, four with positive serum galactomannan, two with air crescent signs, and two with necrotic skin lesions of the palate and nose compatible with Mucoral spp. (Figure 2). Table 3 shows the frequency of IFI in the patients with hematological malignancy.
| No. | Age | Sex | PMH | Clinical Findings | Chemotherapy Course | Neutrophil (mL) | Hb (mg/dL) | Platelet (in mL) | ESR | IFI Sign | Diagnosis Confirmation | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Male | ALL | Fever, dyspnea, cough, pharyngitis | 4 | 50 | 8.2 | 20000 | 70 | None | Aspergillus spp. in BAL | Death |
| 2 | 70 | Male | CLL, DM | Fever, lethargy, abdominal pain, malaise | 3 | 210 | 7.8 | 74000 | 75 | Serum GM | None | Death |
| 3 | 30 | Male | AML | Fever, pharyngitis | 2 | 8400 | 9.5 | 41000 | 54 | Chest nodule on CT | None | Recovery |
| 4 | 60 | Female | AML | Fever, bone pain, weight loss | 7 | 120 | 9.8 | 16000 | 43 | Necrotic lesion | None | Death |
| 5 | 67 | Male | DM, AML | Fever, weight loss, cough, dyspnea, cellulitis | 3 | 4300 | 10.4 | 97000 | 100 | None | Candida spp. in blood culture | Recovery |
| 6 | 70 | Male | AML | Fever, malaise, lethargy, bleeding | 4 | 670 | 7.2 | 6000 | 89 | Necrotic lesion | None | Death |
| 7 | 59 | Male | DM, CLL | Malaise, dyspnea, abdominal pain | 1 | 7600 | 10.4 | 84000 | 17 | Chest nodule on CT | None | Recovery |
| 8 | 29 | Male | ALL | Fever, weight loss, cough, cellulitis | 2 | 16000 | 8.4 | 648000 | 45 | Chest nodule on CT | None | Recovery |
| 9 | 48 | Male | AML | Fever, dyspnea, cough, abdominal pain | 2 | 340 | 8.8 | 3000 | 50 | None | Candida spp. in blood culture | Death |
| 10 | 44 | Female | AML | Fever, malaise | 3 | 5600 | 8.8 | 141000 | 56 | None | Candida spp. in blood culture | Recovery |
| 11 | 86 | Male | MDS | Fever, cough, pharyngitis, dyspnea, lethargy | 2 | 1800 | 8.5 | 50000 | 88 | Serum GM | Aspergillus spp. in BAL | Death |
| 12 | 75 | Male | MDS | Fever, cough, dyspnea, bleeding | 3 | 23000 | 9.2 | 7000 | 115 | Air crescent sign | None | Death |
| 13 | 55 | Male | DM, HD | Fever, bone pain, dyspnea | 3 | 180 | 11.4 | 187000 | 98 | Chest nodule on CT | None | Recovery |
| 14 | 78 | Female | MM | Bone pain, dyspnea, lethargy | 3 | 4300 | 9.8 | 325 | 117 | None | Aspergillus spp. in BAL | Death |
| 15 | 20 | Female | ALL | Fever | 5 | 180 | 8.6 | 9000 | 32 | Chest nodule on CT | None | Death |
| 16 | 52 | Male | DM, NHL | Malaise, cough, dyspnea | 4 | 160 | 9.7 | 44000 | 143 | Chest nodule on CT | None | Death |
| 17 | 20 | Female | HD | Malaise, cough, abdominal pain, seizure, weight loss | 4 | 1870 | 11.5 | 154000 | 54 | Serum GM | None | Recovery |
| 18 | 20 | Male | ALL | Fever, bone pain, malaise, weight loss, lethargy | 3 | 2100 | 7.2 | 313000 | 70 | Chest nodule on CT | None | Recovery |
| 19 | 79 | Female | DM, AML | Dyspnea, lethargy, cellulitis | 3 | 13800 | 8 | 60000 | 40 | Serum GM | None | Death |
| 20 | 50 | Male | DM, AML | Malaise, pharyngitis, lethargy | 7 | 30 | 9.9 | 10000 | 117 | Air crescent sign | None | Death |
| 21 | 62 | Male | AML | Fever, bone pain, malaise, cough, dyspnea | 4 | 1400 | 11.2 | 110000 | 40 | Chest nodule on CT | None | Recovery |
| 22 | 69 | Male | DM, ALL | Fever, bone pain, malaise, weight loss, lethargy | 6 | 350 | 8.8 | 179000 | 65 | None | Aspergillus spp. in BAL | Death |
| 23 | 71 | Male | DM, NHL | Bone pain, weight loss, cough, dyspnea, cellulitis | 6 | 190 | 10.8 | 127000 | 98 | Chest nodule on CT | None | Recovery |
| 24 | 70 | Female | DM, AML | Fever, bone pain, malaise, cough, dyspnea, lethargy, cellulitis | 5 | 50 | 11.2 | 9000 | 110 | Chest nodule on CT | None | Death |
Demographic Characteristics, Clinical Symptoms, and Paraclinical Findings of the Patients with Invasive Fungal Infection
| Group | IFI (n = 24) | Non-IFI (n = 36) | P-Value |
|---|---|---|---|
| Age (mean ± SD ) | 55.9 ± 19.8 | 57.9 ± 15.2 | 0.675 |
| Male/female | 17.07 | 21.15 | 0.416 |
| Fever | 17 | 21 | 0.416 |
| Cough | 11 | 10 | 0.176 |
| Dyspnea | 13 | 9 | 0.030 a |
| Malaise | 11 | 12 | 0.419 |
| Bone pain | 8 | 7 | 0.362 |
| Weigh loss | 7 | 12 | 0.784 |
| Bleeding | 2 | 3 | 1.000 |
| Abdominal pain | 4 | 8 | 0.787 |
| Lethargy | 9 | 12 | 0.702 |
| Pharyngitis | 4 | 4 | 0.702 |
| Cellulitis | 5 | 3 | 0.247 |
| Seizure | 1 | 1 | 1.000 |
| Diabetes mellitus | 10 | 13 | 0.788 |
| Corticosteroid treatment | 13 | 12 | 0.109 |
The Demographic Characteristics and Primary Symptoms of the Patients (n = 60)
| Group | IFI (n = 24) | Non-IFI (n = 36) | P-Value | |
|---|---|---|---|---|
| Primary hematological malignancy | Proven | Probable | ||
| Acute myeloid leukemia | 3 | 7 | 11 | 0.384 |
| Acute lymphoid leukemia | 0 | 5 | 4 | |
| Myelo dysplastic syndrome | 0 | 2 | 6 | |
| Chronic lymphocytic leukemia | 0 | 2 | 5 | |
| Multiple myeloma | 0 | 1 | 5 | |
| Non-hodgkin lymphoma | 0 | 2 | 2 | |
| Chronic myelogenous leukemia | 0 | 0 | 3 | |
| Hodgkin's disease | 0 | 2 | 0 | |
Frequency of Invasive Fungal Infection (IFI) in Patients with Hematological Malignancies
The Kolmogorov-Smirnov test showed that variables such as age, neutrophil count, and number of chemotherapy courses did not distribute normally. The Mann-Whitney U test revealed that the mean neutrophil count was significantly lower in the IFI group compared to the non-IFI group (P = 0.001). Fisher's exact test indicated that severe neutropenia (below 500/mL) was more common in the IFI group (P = 0.002) (Table 4). The three-month mortality rate was significantly higher in the IFI group at 58.3% (14 out of 24 patients) compared to 27.8% (10 out of 36 patients) in the non-IFI group (P = 0.031). However, Fischer's exact test showed that mortality rate did not correlate with the etiology of IFI (P = 0.763) (Table 5).
Mann-Whitney U Test for Comparing Quantitative Data in Invasive Fungal Infection (IFI) and Non-IFI Groups (n = 60)
| Etiology of IFI | Aspergillus spp. | Candida spp. | Mucoral spp. | P-Value |
|---|---|---|---|---|
| Total cases | 19 | 3 | 2 | 0.763 |
| Mortality | 11 | 1 | 2 |
Mortality Rate Associated with Invasive Fungal Infection (IFI) Etiology (n = 24)
5. Discussion
Invasive fungal infection is a relatively common and life-threatening infection in patients with hematological malignancies. Our study found an 11.3% frequency of IFI among hospitalized patients with these conditions. The mean age of patients with IFI was 57 years, with the most common malignancy being AML. The mortality rate for our IFI patients was 58.3%. The most frequently identified fungi were Aspergillus, followed by Candida and Mucoral spp. Severe neutropenia, indicated by a neutrophil count below 500/mL, was the only identified risk factor for IFI in our study.
According to various studies in Iran, IFI is most commonly caused by Candida and Aspergillus spp. A three-year study involving 490 patients with IFI admitted to the ICU reported that 68.8% had Candida spp., 22.1% had Aspergillus spp., and 4.3% had Zygomycetes, based on molecular diagnosis (8). Another single-center, five-year study involving 617 patients with leukemia in Tehran identified 87 cases of IFI using culture, biopsy, and serum galactomannan levels, finding Candida spp. in 74.7%, Aspergillus spp. in 17.2%, and Zygomycetes in 11.5% (9).
The incidence and mortality rates of IFI vary by region. A ten-year study in Japan found a cumulative IFI incidence of 10.5% among patients with hematological malignancies and a mortality rate of 61.2% (10). In China, a study involving 323 patients with hematological malignancies reported an IFI development rate of 3.5%, consisting primarily of Candida and Aspergillus spp. (11). Another Japanese study on 2821 patients showed that 1.3% developed IFI, with 40% mortality during the study period (12). A study from Spain (2004-2015) involving 285 patients noted an IFI frequency of 10% (13).
Our study reported higher frequency and mortality rates compared to these studies but found similar fungal etiologies, with Aspergillus, Candida, and Mucoral spp. being the most common causes of IFI. Other studies also reported IFI frequencies ranging from 5% to 45%. Most patients acquiring IFI were middle-aged, with other studies reporting mean ages from 44 to 61 years. Like our findings, AML was frequently the most common hematological malignancy associated with IFI in these studies (10, 14-17).
As demonstrated in Table 4, severe neutropenia, defined as a neutrophil count less than 500 cells/mL, was identified as the most significant risk factor for IFI in our study. This condition commonly results from chemotherapy, and many studies have confirmed its critical role as a risk factor for IFI. Additionally, various other risk factors for IFI have been identified (18-20). A study involving 102 patients with hematological malignancies in Iran found that being over 60 years old, having diabetes mellitus, a previous history of IFI, receiving more than three types of antibiotics, and undergoing more than eight chemotherapy courses were significant risk factors for IFI (19). Another study from Spain identified corticosteroid treatment and recent viral infection as risk factors in patients with hematological malignancies, with only 12% of these patients being neutropenic (20).
In our patient cohort, the most common causes of IFI were Aspergillus spp., followed by Candida and Mucoral spp. However, the mortality rate among our patients did not correlate with the etiology of the IFI. Previous research has shown varying incidence and mortality rates of IFI in patients with hematological malignancies depending on the fungal etiology. In one study from India, a 10% incidence of candidemia was reported among 150 patients with hematological malignancies associated with acute lymphoblastic leukemia (ALL), leukopenia, long-term intravenous catheter use, and corticosteroid treatment (21). A multicentric study in Greece between 2009 and 2012 reported a candidemia incidence rate of 0.014% among patients admitted with hematological malignancies (22). In Italy, 215 episodes of IFI were recorded among patients with hematological malignancies, with Candida spp. being the predominant cause and associated with a 39% mortality rate (23). A German study conducted from 2003 to 2009 found an annual rate of 1.1 per thousand hospitalizations for candidemia, accompanied by a 67% three-month mortality rate (24).
Several studies have documented an increased frequency of pulmonary aspergillosis in patients with hematological malignancies. For instance, an Italian study reported 61 cases of invasive pulmonary Aspergillus (IPA) among patients with hematological malignancies, where the incidence rate was 7.1% and the three-month mortality rate was 27%. The most common underlying malignancy in these cases was AML (25). Similarly, a 20-year study in France from 1998 to 2017 identified 217 patients with IPA, again with AML as the most frequent underlying disease and a three-month mortality rate of 75% (26).
Other studies have also noted an increased rate of various invasive fungi in this patient population. A five-year study involving 2083 patients with hematological malignancies in China found that 11.3% had IFI, caused by Aspergillus spp., Cryptococcus, and Mucor, respectively. AML was the most common underlying malignancy here as well, with a three-month mortality rate from IFI at 5.9% (27). In our study, dyspnea was more prevalent among patients with IFI than those without, potentially due to the higher incidence of IPA in the IFI group.
Our findings indicate a three-month mortality rate of 58.3% for IFI, aligning with other studies showing mortality rates ranging from 5% to 75% (3, 4, 10, 16, 17, 22, 26-28), influenced by factors such as underlying malignancies, chemotherapy regimens, comorbidities, surgical interventions, and history of antifungal prophylaxis. Uniquely, our study identified two cases (8.3%) of facial and oral necrotic lesions likely due to Mucoral spp., both of which were fatal. A study in Egypt on children with cancer associated invasive fungal sinusitis with a 35% three-month mortality rate (29).
We recommend that future studies further investigate IFI with respect to types of hematological malignancy, chemotherapy regimens, and antimicrobial prophylaxis strategies. There is also a need for enhanced laboratory capabilities to facilitate the diagnosis of fungal infections, including fungal sub-typing and antifungal susceptibility testing, in all tertiary hospitals or treatment centers for these patients.
5.1. Conclusions
Our study identified a frequency of 11.3% of IFI and a three-month mortality rate of 58.3% among patients with hematological malignancies. Aspergillus and Candida spp. were the most frequently identified fungi, consistent with previous studies, and AML was the most common underlying malignancy. We recommend that all medical centers with hematology departments implement advanced laboratory systems for the diagnosis and identification of invasive fungal subspecies and antifungal drug sensitivity testing. However, our study faced several limitations:
- Absence of histopathological and microscopical confirmation of fungal infections.
- Lack of fungus sub-typing and antifungal susceptibility tests.
- Unavailability of data on antimicrobial prophylaxis, such as cotrimoxazole, which could prevent Pneumocystis jirovsi, preventing a comprehensive analysis of the relationship between antimicrobial prophylaxis and IFI.