1. Background
Candida albicans is the most common pathogen responsible for the development of oral candidiasis (1-3). Oral candidiasis can cause chronic pain and discomfort during mastication and complicate food intake, particularly in the elderly and immunocompromised patients (4, 5). The empirical use and overuse of antifungal agents in clinical and hospital settings have led to the development of resistant species, especially in immunocompromised patients (6). Infections caused by resistant microorganisms often do not respond to commonly prescribed antifungal agents and tend to have a prolonged course (6, 7). Antifungal resistance in C. albicans is an emerging global dilemma (7, 8). Without treatment, ineffective treatment, or in cases of compromised immunity, the infection can spread locally to the esophagus or systemically through the bloodstream, leading to candidemia, which is associated with high morbidity and mortality rates (9). Oral candidiasis affects approximately 5 - 7% of infants, with an estimated prevalence of 20% in cancer patients (4). It also has a prevalence of 39% in patients with acquired immunodeficiency syndrome in Asia (10).
The emerging resistance of bacteria and fungi to antibiotics and antifungal agents has led to the development of new bacteriostatic, bactericidal, and fungicidal treatments (11, 12). Laser therapy is one of these new approaches, offering several competitive advantages over traditional drug treatments. Unlike treatment with antiseptics and antibiotics, laser therapy does not require high concentrations of medications at the target site, making it effective for recurrent superficial mucosal or cutaneous infections (13). Laser treatment has a different mechanism of action that can reduce the likelihood of resistance. Adjusting the energy delivery rate and combining wavelengths have been shown to delay resistance formation (14). Additionally, lasers provide a localized, non-invasive treatment option that precisely targets infected areas without affecting surrounding tissues. This precision is particularly beneficial in treating biofilms and mucosal infections where systemic antifungal agents may be less effective (15, 16).
The antimicrobial activity of lasers appears to be related to thermal effects and photo-disruption (17). However, Najafi et al. (13) found that laser therapy can sometimes increase the proliferation of bacteria and fungi. A few other studies have shown that laser therapy did not significantly affect bacteria and fungi, either qualitatively or quantitatively (18, 19). Diode lasers with wavelengths of 660 nm, 810 nm, and 940 nm are commonly used in medical applications (20-24). The 660 nm diode laser has shown significant antimicrobial activity, making it valuable in treating surface infections. It can also be combined with photosensitizers in photodynamic therapy to enhance antimicrobial effects (22-24). The 810 nm and 940 nm diode lasers have demonstrated antimicrobial applications, particularly in dentistry, due to their ability to generate heat and penetrate deeper than shorter wavelengths, such as the 660 nm laser (16, 20-22, 25). However, the evaluation of 660 nm, 810 nm, and 940 nm wavelengths in the context of nystatin-resistant C. albicans strains has not been extensively studied.
2. Objectives
Given the existing controversy regarding the effects of laser irradiation at different wavelengths on C. albicans and the widespread use of 660 nm, 810 nm, and 940 nm diode lasers in medicine (13, 18, 19, 26), this in-vitro study aimed to assess the effects of 660, 810, and 940 nm laser wavelengths on nystatin-resistant C. albicans.
3. Methods
3.1. Preparation of Candida albicans Strains Suspension
The C. albicans samples included a standard strain (ATCC 10221) and eight clinical isolates of nystatin-resistant C. albicans, obtained from patients diagnosed with blood cancer and suffering from denture stomatitis. Drug susceptibility testing confirmed nystatin resistance in the isolates. All samples were stored in the repository of the Department of Medical Mycology in Iran and were accessible for research purposes. In this study, 720 samples of 9 different strains (10 groups of each, with 8 repetitions) were evaluated.
Candida albicans strains were cultured on Sabouraud dextrose agar (SDA; Sigma-Aldrich, USA). Then, pure colonies from the SDA medium were isolated using a glass loop and transferred to a tube containing 2 mL of physiological saline solution (0.9%). A suspension was then prepared with a 0.5 McFarland standard concentration (equal to 0.5 × 106 CFUs/mL) in microtubes containing 5 mL of distilled water. The turbidity of the cell suspensions was measured with a spectrophotometer at 530 nm.
Afterward, 100 µL of the suspension for each isolate was added to a microplate. Additionally, 100 µL of saline was added to the laser and control wells, while the same amount of nystatin (100,000 units/mL; Hakim Pharmaceuticals, Iran) was added to the wells in the nystatin group. The microplate height from the bottom to the surface was 1 cm, and for laser irradiation, the probe was placed in contact with the microplate. The microplates were then irradiated with different laser doses for varying periods. All procedures were performed under a laminar hood to maintain sterile conditions. After laser irradiation, the suspensions were added to the culture medium, and the plates were incubated at 37°C for 48 hours. The number of colonies was then counted, and nystatin susceptibility was evaluated using the minimum inhibitory concentration (MIC) (27).
3.2. Laser Parameters
Four different diode lasers were utilized in this study: 1. 660 nm (Hammers; 3L-R, Iran) with a red probe, 2. 810 nm (GaAlAs; Thor, UK) with a single infrared probe, 3. 810 nm (Photon, Zolar, Canada) with a cutting fiber probe, and 4. 940 nm (InGaAsP Biolase Epic 10, USA) with a deep tissue handpiece. The diameter of all probes was 1 cm, and they were calibrated before use by measuring the output power with a power meter. The lasers were applied with an energy density of 20.38 J/cm2 (ED = P.t/A) in continuous-wave mode. The peak power was set at 200 mW and 400 mW. Specimens were irradiated with the laser for 40 and 80 seconds (28).
The study groups are presented in Table 1.
| No. | Groups |
|---|---|
| 1 | 40-second, 400 mW 660 nm laser irradiation |
| 2 | 80-second, 200 mW 660 nm laser irradiation |
| 3 | 40-second, 200 mW 810 nm GaAlAs laser irradiation |
| 4 | 80-second, 400 mW 810 nm photon laser irradiation |
| 5 | 40-second, 200 mW 940 nm laser irradiation |
| 6 | 80-second, 400 mW 940 nm laser irradiation |
| 7 | 40-second nystatin |
| 8 | 80-second nystatin |
| 9 | Positive control (standard-strain Candida albicans without laser irradiation) |
| 10 | Negative control (only culture medium) |
3.3. Candida albicans Colony Count
Following laser irradiation, approximately 100 µL of the irradiated C. albicans suspension (with a concentration of 0.5 × 106 CFU/mL) was applied to an SDA plate. Using a glass spreader, the cells were evenly distributed across the plate. The plates were then incubated at 37°C for 24 hours, and the colony count on each plate was determined. Colony counting was performed in duplicate.
3.4. Minimum Inhibitory Concentration
After laser irradiation, C. albicans suspensions (standard strain and clinical isolates), prepared according to the M27-A3/S4 Clinical Laboratory Standards Institute protocol, were used for nystatin susceptibility testing. For this purpose, the Roswell Park Memorial Institute culture medium (RPMI; Sigma-Aldrich, USA) was first prepared as instructed in the brochure. Specifically, 11.4 g of RPMI powder and 34.5 g of morpholine propane sulfonic acid were added to 1 L of distilled water, with the pH of the culture medium adjusted to 6.98 - 7.02. A vacuum pump was then used to filter the solution. The filtered RPMI was stored in glass vials and refrigerated until use.
After preparing the culture medium, a yeast suspension of laser-irradiated colonies was made using sterile distilled water at a 0.5 McFarland standard concentration. Next, 100 µL of this suspension was added to microplate wells. Serially diluted nystatin (100,000 units/mL; Hakim Pharmaceuticals, Iran) was added to the wells such that the concentration of nystatin was 8 µg/mL in the first well, decreasing to 4, 2, 1, 0.5, 0.25, 0.12, 0.06, 0.03, and 0.015 µg/mL in subsequent wells. The microplates were then incubated at room temperature for 24 hours, and the MIC was recorded for each group. A minimum inhibitory concentration less than 1 indicates susceptibility to nystatin, an MIC greater than 2 indicates resistance, and an MIC between 1 and 2 indicates intermediate susceptibility of C. albicans isolates to nystatin (29).
3.5. Statistical Analysis
Data were analyzed using SPSS 26. A one-sample t-test was employed to compare the intervention groups with the control group. An independent samples t-test was applied to compare the 40- and 80-second irradiation times. One-way ANOVA, followed by Tukey’s HSD test, was used for comparisons among intervention groups.
4. Results
4.1. Candida albicans Colony Count
4.1.1. Standard-Strain Candida albicans
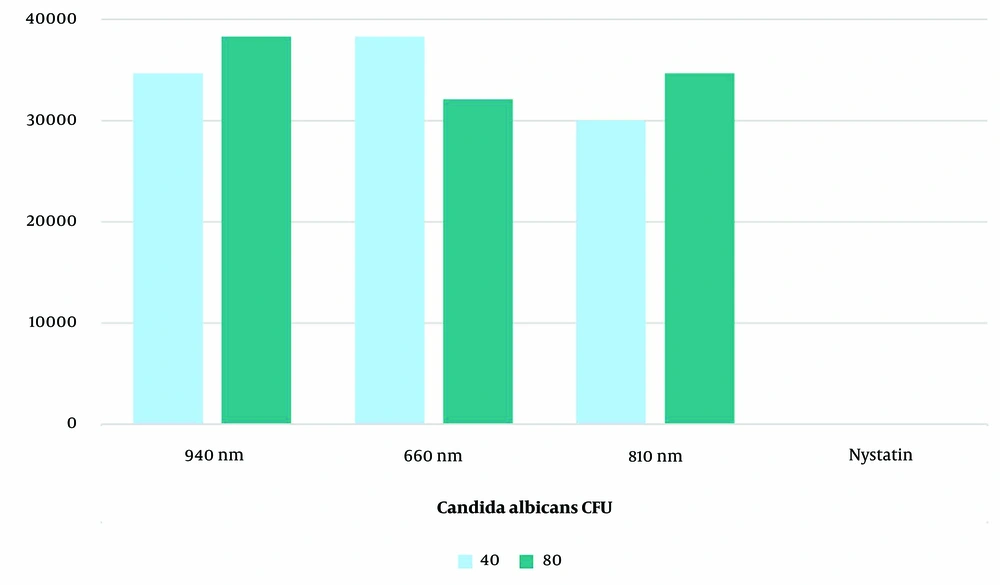
The evaluation of the standard strain of C. albicans colony count revealed that the nystatin group showed the most substantial reduction after both 40 and 80 seconds of exposure, reducing the count to zero (Figure 1). This group had a significantly lower mean colony count compared to all laser groups at both exposure times (P < 0.001). Among the laser groups, the 810 nm laser for 40 seconds caused the greatest reduction, followed by the 660 nm laser for 80 seconds.
All groups (laser and nystatin) demonstrated a significant decrease in C. albicans colony count compared to the positive control group (P < 0.001). Time (P = 0.012), group (P = 0.001), and their interaction (P = 0.001) were identified as significant factors affecting colony count according to the between-subject effects test. Independent analysis of the time effect showed no significant difference between 40 and 80 seconds of exposure in the nystatin and 940 nm laser groups (P = 0.066). In the 810 nm laser group, 40 seconds of irradiation (P = 0.006), and in the 660 nm laser group, 80 seconds of irradiation (P = 0.002), resulted in a significantly greater reduction in colony count. After 40 seconds of exposure, the mean colony count in the 810 nm laser group was significantly lower than in the 660 nm (P < 0.001) and 940 nm (P = 0.014) laser groups. However, the difference between the 660 nm and 940 nm laser groups was not significant (P = 0.057). After 80 seconds of exposure, the mean colony count in the 660 nm laser group was significantly lower than in the 940 nm laser group (P = 0.001), while no significant difference was observed between the 810 nm laser group and the 660 nm (P = 9.342) or 940 nm (P = 0.073) laser groups.
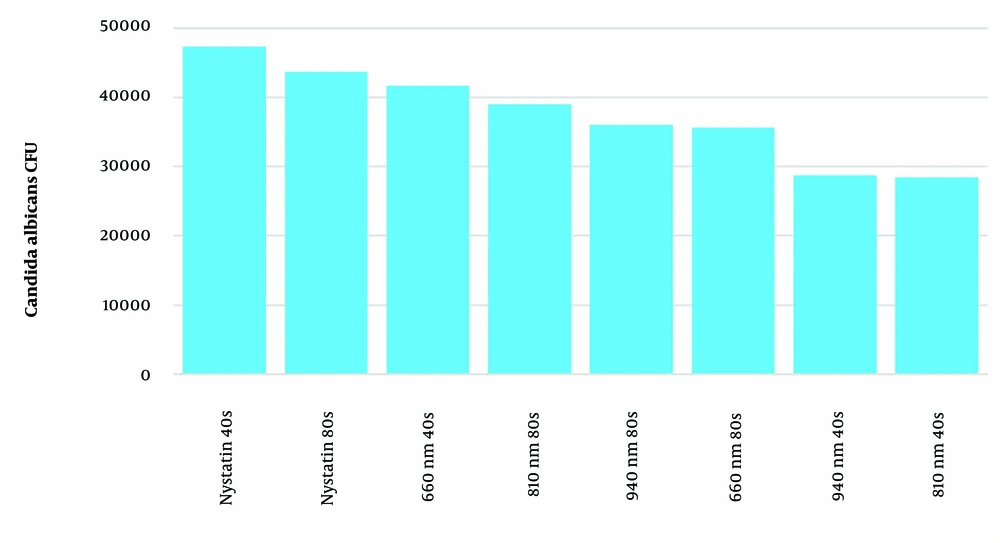
4.1.2. Nystatin-Resistant Candida albicans Clinical Isolates
Figure 2 shows the mean colony count of nystatin-resistant C. albicans clinical isolates in the different examined groups. All groups, including laser and nystatin treatments, exhibited a significant reduction in the colony count of nystatin-resistant C. albicans compared to the positive control group (P < 0.001). The analysis revealed significant effects of time (P < 0.001), group (P < 0.001), and their interaction (P < 0.001) on colony count. In terms of exposure time, 80 seconds in the nystatin and 660 nm laser groups led to a significantly greater reduction compared to 40 seconds (P < 0.001), whereas 40 seconds of exposure in the 810 nm and 940 nm laser groups resulted in a significantly greater reduction compared to 80 seconds (P < 0.001).
After 40 seconds of exposure, the mean colony count in the 810 nm and 940 nm laser groups was significantly lower than in the 660 nm laser and nystatin groups (P < 0.001). The mean colony count in the 660 nm laser group was significantly lower than in the nystatin group but higher than in the 940 nm laser group (P < 0.001). However, the difference between the 810 nm and 940 nm laser groups was not significant (P = 0.991). After 80 seconds of exposure, the maximum reduction was observed in the nystatin group. The mean colony count in the 660 nm laser group was significantly lower than in the 810 nm laser (P = 0.004) and nystatin (P < 0.001) groups. Similarly, the mean colony count in the 940 nm laser group was significantly lower than in the 810 nm laser (P = 0.026) and nystatin (P < 0.001) groups, and the 810 nm laser group also showed a significantly lower mean colony count than the nystatin group (P < 0.001). However, the difference between the 660 nm and 940 nm laser groups was not significant (P = 9.942).
4.2. Minimum Inhibitory Concentration Results
Irradiation with the 660 nm laser for 80 seconds had the least effect on the MIC among the laser groups. Irradiation with the 810 nm laser, followed by the 940 nm laser, both for 40 seconds, had the greatest effect on the MIC. However, only irradiation with the 810 nm laser decreased resistance to nystatin in the standard strain of C. albicans (Table 2).
| Isolates | Before Laser Irradiation | After 940nm Laser Irradiation | After 810nm Laser Irradiation | After 660nm Laser Irradiation | |||
|---|---|---|---|---|---|---|---|
| - | 80s | 40s | 80s | 40s | 80s | 40s | |
| 1 | 4 | 4 | 1 | 2 | 2 | 4 | 4 |
| 2 | 4 | 4 | 1 | 2 | 2 | 4 | 4 |
| 3 | 2 | 4 | 1 | 2 | 4 | 2 | 4 |
| 4 | 4 | 4 | 1 | 2 | 2 | 2 | 4 |
| 5 | 2 | 4 | 1 | 2 | 2 | 2 | 4 |
| 6 | 2 | 4 | 1 | 2 | 2 | 4 | 4 |
| 7 | 2 | 4 | 1 | 2 | 4 | 4 | 4 |
| 8 | 4 | 4 | 1 | 2 | 2 | 4 | 4 |
| Standard strain | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 |
5. Discussion
This study aimed to assess the effects of 660 nm, 810 nm, and 940 nm laser wavelengths in comparison with nystatin on nystatin-resistant C. albicans under in vitro conditions. The results showed that all laser wavelengths, irradiated for 40 and 80 seconds, significantly decreased the colony count of the standard strain of C. albicans. Mardani and Kamrani (25) evaluated the effect of 810 nm laser irradiation (300 mW power, 228 J/cm² energy density) on standard-strain and fluconazole-resistant C. albicans, showing that laser irradiation significantly decreased the colony count of both strains compared to the control group, which aligns with the present findings. Similarly, Maver-Biscanin et al. (30) demonstrated that 685 nm (30 mW power) and 830 nm (60 mW power) laser irradiation reduced denture stomatitis in patients.
In a subsequent study, Maver-Biscanin et al. (31) evaluated the efficacy of low-level laser irradiation with an 830 nm wavelength (3 J/cm², 60 mW, 5 minutes) and a 685 nm wavelength (3 J/cm², 30 mW, 10 minutes) in patients with denture stomatitis and reported a reduction in colony count and palatal inflammation (31), partially supporting the present results. Souza et al. (32) reported a reduction in C. albicans colony count using a 660 nm low-level laser (GaAlAr). Daliri et al. (33) also demonstrated that 660 nm diode laser irradiation (10 mW and 100 mW powers) and 460 nm laser (25 mW power) for 30 and 60 seconds significantly decreased the colony count of the standard strain of C. albicans compared to the control group. Both studies’ results agree with the present findings.
Guffey et al. (26) used a 624 nm laser with energy densities of 3, 9, and 30 J/cm² and reported results similar to the present study. In their study, energy densities of 15 and 60 J/cm² showed no significant difference from the control group. They also noted that the presence of a photosensitizer is not always necessary for fungal control, adding that high energy density is also not required, making this modality suitable for clinical use. Queiroga et al. (34) compared the effects of a 660 nm laser with energy densities of 60, 120, and 180 J/cm², applied for 1, 2, and 3 minutes, on various Candida species and showed that the 660 nm laser with all three energy densities significantly decreased C. albicans colony count. Their laser wavelength was similar to the one used in the present study.
Wiench et al. (35) evaluated the biofilm of different Candida species, including C. albicans, C. glabrata, and C. krusei, and indicated that irradiation with a 635 nm diode laser (400 mW, 24 J/cm² energy density, 30 seconds) significantly decreased the colony count of C. albicans. However, Najafi et al. (13) reported that irradiation with a 940 nm laser at energy densities of 38 and 76 J/cm² for 30 and 60 seconds increased the colony count of C. albicans, which differs from the present findings.
The effect of low-level laser on C. albicans depends on various factors such as energy dosage, power, power density, wavelength, mode of laser application (pulse or continuous, contact or non-contact), contamination with other microorganisms, and irradiation time (13). In the study by Najafi et al. (13), the use of high-power laser, which increased ATP production in Candida, resulted in lower degradation of fungal cells. This could explain the difference in results between the two studies. Hamblin et al. (18) evaluated the effects of 685 nm and 830 nm laser wavelengths with energy densities of 6, 8, 10, and 12 J/cm² on clinical isolates from immunosuppressed patients and found no significant effect. The maximum reduction in C. albicans colonies was observed after irradiation with the 830 nm laser at 6 J/cm² energy density. The difference between their results and the present findings, despite the use of similar laser wavelengths, may be attributed to the lower energy density used in their study.
In the present study, the MIC of nystatin was evaluated after laser irradiation of nystatin-resistant C. albicans clinical isolates. The results showed that irradiation with the 810 nm laser for 40 seconds yielded the most favorable outcomes, with the MIC of nystatin found to be 1 µg/mL for all nystatin-resistant isolates. This result may be attributed to several factors. First, the absorption of light by biological tissues varies with wavelength, and the 810 nm wavelength may have a better absorption coefficient than the 940 nm and 660 nm wavelengths, resulting in more effective energy delivery. Additionally, the 810 nm wavelength falls within the near-infrared range, which is known for its strong photothermal effect, allowing light to be efficiently converted into heat, leading to increased destruction of Candida isolates (13, 18, 19, 36, 37).
While the 940 nm wavelength has deeper tissue penetration than the 810 nm, this may not necessarily be advantageous for treating Candida infections. If the light penetrates too deeply, it may not be absorbed effectively by the fungi, leading to less efficient treatment. Furthermore, the 660 nm wavelength, being in the visible red range, may not penetrate as deeply as the 810 nm wavelength, resulting in less effective treatment. The 810 nm wavelength seems to strike a balance between tissue penetration and absorption efficiency (16, 20-22, 25, 37). The efficacy of laser treatment for eliminating C. albicans resistance has not been extensively studied, making this research novel in that aspect. However, some studies have reported a reduction in the MIC of amphotericin B and fluconazole against different resistant Candida species after exposure to gold, silver, and selenium nanoparticles (1, 38). Additionally, this study's results showed that lasers perform better in treating nystatin-resistant C. albicans strains, which may be due to their non-specific mechanisms of action, ability to alter gene expression, and synergistic effects with antifungal agents (39-43).
Oxidative stress caused by reactive oxygen species (ROS) and singlet oxygen disrupts cell integrity and leads to cell damage and death through necrosis and apoptosis by damaging DNA, proteins, and other intracellular macromolecules (44, 45). Fungal cells are aerobic and naturally produce ROS through mitochondrial metabolism and peroxisomal reactions, producing H2O2 in oxidase-accelerated processes. Endogenous ROS spreads from the mitochondrial electron transport chain through the cell membrane and attacks other organelles and cellular components. The oxidative stress induced by endogenous ROS leads to DNA damage, including strand breaks and purine-pyrimidine breakdown, which can be toxic or mutagenic. Both endogenous ROS and those produced by light are responsible for the genetic damage in C. albicans cells (34). Furthermore, it has been reported that low-level laser irradiation induces polymorphonuclear leukocytes to produce higher levels of ROS, such as hypochlorite anion and hydroxyl radicals (39), enhancing their antimicrobial activity and fungicidal effect on C. albicans (39, 40).
It is believed that low-level laser irradiation degrades the cell walls of microorganisms, leading to the accumulation of denatured proteins in the cytoplasm, causing damage and death. Under such conditions, microorganisms attempt to maintain homeostasis despite the accumulation of stress, resulting in inhibited proliferation and cell lysis. Altered interactions between the microorganism and the cell substrate may also disrupt critical activities (46). Laser light modulates transmembrane heat transfer and may cause degradation of treated cells, a process explained by the shrinkage and expansion of the intercellular volume of water, creating two-way water flow through the cell membrane (41). One theory regarding the mechanism of low-level laser therapy suggests that the laser affects the endogenous chromophores of fungi, inhibiting their adhesion and dimorphism, which decreases the virulence of Candida (42).
The use of low-level laser without a photosensitizer can effectively eradicate superficial fungal infections (43). Laser therapy accelerates the electron transport chain in the mitochondria, leading to increased ATP production (39). Additionally, the co-stimulation of the immune response through ATP production in the mitochondria should be considered. Under in vitro conditions, laser light is absorbed only by C. albicans, and its effects on human immune cells are not evaluated (47), which is also a limitation of the present study. Given the optimal efficacy of 810 nm laser irradiation in inhibiting C. albicans clinical isolates, as compared to nystatin and standard-strain C. albicans, future studies are recommended to further assess the efficacy of this specific laser wavelength.
5.1. Conclusions
Low-level laser irradiation can effectively reduce the colony count of nystatin-resistant C. albicans. Irradiation with an 810 nm laser at 400 mW power for 40 seconds is the most efficient protocol for this purpose, followed by a 940 nm laser at 400 mW power for 40 seconds, with the same energy density of 20.38 J/cm2. Additionally, assessing the susceptibility of C. albicans to nystatin after laser irradiation was a strength of this study, revealing that all nystatin-resistant C. albicans isolates lost their resistance to nystatin after irradiation with the 810 nm laser for 40 seconds.