1. Background
Sickle cell disease (SCD) constitutes a group of genetic disorders characterized by abnormal hemoglobin structure, resulting in intermittent formation of sickle-shaped red blood cells (RBCs) and diverse clinical manifestations. It impacts thousands of people in Iran (1) and millions globally, predominantly those of black African and Afro-Caribbean descent, as well as individuals from the Mediterranean, Middle East, and parts of India (2). The clinical manifestations of SCD emanate from two primary pathological processes: Vaso-occlusion and hemolysis. Sickle cells, in conjunction with non-sickled RBCs, leukocytes, and platelets, form heterocellular aggregates that adhere to the vascular endothelium, leading to luminal obstruction in small blood vessels. This microcirculatory occlusion precipitates acute and chronic tissue ischemia and infarction, exerting multisystem effects, particularly in the bone, lungs, brain, kidneys, and spleen. Sickled RBCs, owing to their rigidity and changes in the lipid bilayer structure, are more susceptible to destruction by the reticuloendothelial system, primarily in the spleen, fostering phagocytosis (3).
Sickle cell disease patients exhibit susceptibility to infections attributable to impaired splenic function, defects in complement activation, deficiencies in micronutrients, genetic factors, and mechanical factors. While all these factors contribute to viral infection progression, mechanical factors play a crucial role in the susceptibility of SCD patients to viruses such as primate erythroparvovirus 1 (known as B19). Sickle cell disease patients are predisposed to B19, as well as other blood-borne viruses, mainly through iatrogenic infections resulting from blood product transfusion (4). However, in countries with B19 screening programs for blood products, respiratory transmission is the major route of infection (5).
Parvovirus B19, the etiological agent of erythema infectiosum (fifth disease) in children, demonstrates a tropism for erythroid progenitor cells, temporarily suppressing erythropoiesis in the bone marrow. Parvovirus B19 infection exhibits a global epidemiological presence, as evidenced by widespread IgG antibodies in individuals from the United States, Europe, and Asia. The global prevalence of anti-B19 IgG antibodies ranges from 2 to 15% in 1 to 5 years old, 15 to 60% in 6 to 19 years old, 30 to 60% in adults, and exceeds 85% in the senescent population (6).
In Iran, the seroprevalence of anti-B19 IgG antibodies and DNA prevalence in the general population are reported up to 86.6% and 10.8%, respectively (7). In healthy subjects, B19 infection may induce mild pancytopenia, but these alterations are transient, with spontaneous recovery. Conversely, in SCD and Thalassemia patients, parvovirus B19 infection manifests as a more severe condition, termed transient aplastic crisis, characterized by bone marrow hypocellularity alongside the presence of two or more cytopenias, including a reticulocyte count of less than 1% or less than 40,000 per microliter, neutropenia of less than 500 per microliter, or thrombocytopenia of less than 20,000 per microliter (8).
A closely related virus, namely human parvovirus 4 (P4), first identified in 2005 in the serum of intravenous drug users infected with hepatitis B virus, shares some key similarities in epidemiological properties with B19. This virus is a single-stranded non-enveloped DNA virus belonging to the Parvoviridae family and Tetraparvovirus genus (9). Despite having key similarities with B19, which will be scrutinized in the discussion section, P4 lacks established global epidemiological data, clinical manifestations, pathogenesis, and immunogenesis. In existing limited epidemiological studies, anti-P4 IgG antibodies and P4 DNA are found in up to 49% and 17%, respectively, of healthy Iranian individuals (10, 11).
There are no global reports on the prevalence of P4 in healthy populations. Nevertheless, this virus has been detected in various biological samples, including blood products (12), autopsy specimens (13), respiratory samples (14), and cerebrospinal fluid (CSF) (15). However, the causal relationship between P4 and observed diseases remains uncertain, prompting exploration of whether potential risk groups such as SCD patients are at risk for P4 and inquiries into the prevalence and manifestations of P4 in comparison to B19.
2. Objectives
The present study aims to evaluate the prevalence and manifestations of P4, and the co-prevalence of this virus with B19 in SCD patients referred to Shahid Baqaei 2 Hospital in Ahvaz, Iran, during the year 2023.
3. Methods
A total of 165 participants were enrolled, comprising 120 individuals diagnosed with SCD and 45 healthy subjects serving as the control group. The determination of sample sizes adhered to Cochran's formula. All SCD patients presenting at Shahid Baqaei 2 Hospital, who provided informed consent for blood sample collection and study participation, were included. The inclusion criteria for the healthy control group, alongside obtaining consent, encompassed non-pregnancy, absence of chronic diseases, no current active infections, no ongoing medication use, absence of symptoms, absence of immunocompromising conditions such as AIDS, and no history of hepatitis. Whole blood samples from study participants were collected in EDTA tubes and stored at -70°C throughout the study's execution. Viral nucleic acid extraction was performed using the commercial SinaClon Viral Nucleic Acid Extraction Kit (SinaClon, Iran). The concentration of single-stranded DNA in the samples was determined using the NanoDrop Microvolume Spectrometer (Thermo Scientific, Germany). The amplification of viral DNA was accomplished through the nested PCR method, utilizing Taq DNA Polymerase 2x Master Mix RED and 1.5 mM MgCl2 (AMPLICON, Denmark), along with primers specified in Table 1.
| Viruses | PCR Stage | Primer Sets | Annealing Temp. (°C) | Product Length | Reference |
|---|---|---|---|---|---|
| Human parvovirus 4 | Round 1 | 5´-AAGACTACATACCTACCTGTG -3´ 5´- GTGCCTTTCATATTCAGTTCC -3´ | 55 | 220bp | (16) |
| Round 2 | 5´- GTTGATGGYCCTGTGGTTAG -3´ 5´-CCTTTCATATTCAGTTCCTGTTCAC-3´ | 57 | 160bp | (16) | |
| Erythroparvovirus 1 | Round 1 | 5'-CAAAAGCATGTGGAGTGAGG-3' 5'-CTACTAACATGCATAGGCGC-3' | 55 | 398 | (17) |
| Round 2 | 5'-CCCAGAGCACCATTATAAGG-3' 5'-GTGCTGTCAGTAACCTGTAC-3' | 57 | 288 | (17) |
The Characteristics of the Primers Used to Perform Nested PCR and Thermocycler Setup of Each Stage
The PCR setup involved two rounds. For Parvovirus 4, Round 1 consists of an initial denaturation at 95°C for 9 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute, with a final extension at 72°C for 7 minutes. Round 2 for Parvovirus 4 maintains a similar setup but with an annealing temperature of 57°C. For Parvovirus B19, both Round 1 and Round 2 involve an initial denaturation at 95°C for 9 minutes, followed by 30 cycles of denaturation at 94°C for 1 minute, annealing at 55°C in Round 1 and 57°C in Round 2 for 2 minutes, and extension at 72°C for 3 minutes, with a final extension at 72°C for 5 minutes.
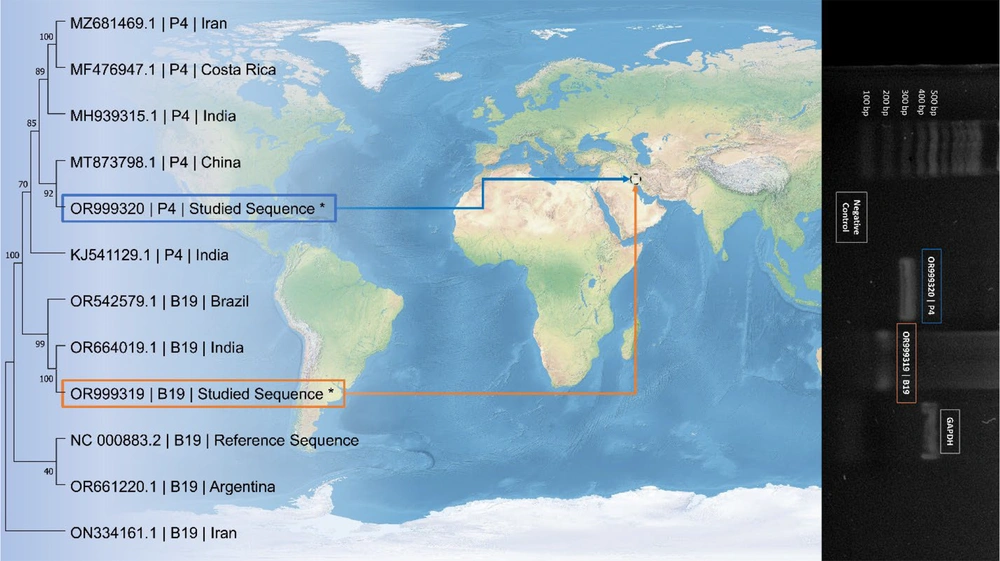
A positive sample containing both viruses was initially acquired from pooled patient samples at the inception of the study. Subsequently, the affirmative outcome underwent Sanger dideoxy sequencing using the ABI Prism 3100 analyzer (Applied Biosystems, USA) and was analyzed through the basic local alignment search tool (BLAST) to confirm the presence of the targeted viruses. The sequencing results have been deposited in the National Center for Biotechnology Information (NCBI) GeneBank, the European Nucleotide Archive (ENA), and the DNA Data Bank of Japan (DDBJ) under the accession numbers OR999319 and OR999320. These sequenced samples were employed as positive controls throughout the study, complemented by the inclusion of negative and internal controls, as depicted in Figure 1.
The phylogenetic tree, illustrated, was constructed using Molecular Evolutionary Genetics Analysis (MEGA) software version 11, employing the Maximum Likelihood statistical method, 100 bootstrap replications, and the Tamura-Nei substitution model. Statistical analyses of the data were conducted in the R programming language version 4.3.2, encompassing basic descriptive methods, the N-1 chi-square test, and the t-test. A 95% confidence interval and a significance level (P-value) of < 0.05 were applied.
4. Results
The mean age within the SCD patient group and the healthy control group was 42.1 ± 12.4 and 39.6 ± 16.1, respectively. The male-to-female sex ratio in the SCD patient group was 52/68, while in the healthy control group, it was 25/20. No statistically significant differences were observed in both mean age and sex distribution between the two groups. Among the SCD patients, 20 individuals (16.6%) with a mean age of 43.8 ± 13.3 and a sex ratio of 10/10 were positive for P4, while 19 individuals (15.8%) with a mean age of 45 ± 13.2 and a sex ratio of 9/10 were positive for B19. In the healthy population, 4 males (8%) with a mean age of 34.8 ± 28.9 were infected with P4, and 2 males (4%) with a mean age of 25 were infected with B19. Additionally, 8 SCD patients (6%) with a mean age of 44.1 ± 14.8 and a sex ratio of 3/5 were co-infected with both viruses. No instances of co-infection were observed in the healthy population. The presence of P4, B19, or co-infection did not alter the mean age and sex distribution across all study groups. However, the prevalence of P4 and B19 was significantly higher in SCD patients compared to healthy individuals, with P-values of 0.0120 and 0.0057, respectively (Table 2).
| Group | Total Patients | Mean Age | Sex Ratio (Male/Female) | Human Parvovirus 4Positive (%) | Erythroparvovirus 1 Positive (%) | Co-infected (%) |
| Sickle cell disease patients | 120 | 42.1 ± 12.4 | 52/68 | 16.6 | 15.8 | 6 |
| Healthy controls | 45 | 39.6 ± 16.1 | 25/20 | 8 | 4 | 0 |
| P-value | - | 0.291 | - | 0.012 | 0.005 | 0.093 |
Comparison of Characteristics and Human Parvovirus 4 and Erythroparvovirus 1 Infection Rates Between Sickle Cell Disease Patients and Healthy Controls
Among the SCD patients, 11 experienced ongoing aplastic crises, with 10 of these cases occurring in B19-infected individuals and 6 in co-infected patients. Despite the majority of aplastic crisis instances being observed in patients with co-infection, statistical analyses indicate that aplastic anemia was not significantly associated with co-infection. Figure 1 illustrates a phylogenetic tree incorporating positive results obtained from pooled samples of SCD patients.
This figure demonstrates a phylogenetic tree including positive results of this study for parvovirus 4 (P4) and primate erythroparvovirus 1 (B19). The studied sequences are denoted red rectangle for B19 and Blue rectangle for P4 and are compared with sequences from studies on the viruses in different clades from different regions of the world. The accession number of the sequences, the name of the virus, and the region the viruses were studied are shown on the phylogenetic tree. On the right side of the picture, there is a photograph of the positive results obtained from patients, a negative control, the housekeeping gene, and ladder bands used during the study.
5. Discussion
This study represents the inaugural attempt to elucidate the prevalence of P4 in individuals afflicted with SCD and its potential impact on aplastic crises. The findings underscore a significantly heightened prevalence of both P4 and B19, as well as their co-occurrence, among SCD patients compared to their counterparts in good health. Notably, the investigation into aplastic crises, a paramount concern associated with B19 in SCD patients, revealed no substantial correlation with either B19-P4 co-infection or P4 alone. However, a higher proportion of patients experiencing recent aplastic crises exhibited co-infection, prompting the imperative need for more expansive studies involving larger populations.
Erythroparvovirus 1, a well-recognized blood-borne virus and a long-standing threat to individuals with SCD, is presently subject to screening in all administered blood products in developed countries. Nevertheless, global infection rates in SCD patients persist between 37.6 to 65.9% based on IgG, 2.9 to 30% based on IgM, and 4 to 54% based on DNA presence, primarily attributed to respiratory droplet transmission (5, 18). Another meta-analysis by Obeid Mohamed et al. published in 2019 indicated a 48.8% seroprevalence for anti-B19 IgG and 8.30% for IgM in SCD patients, with a higher proportion of the infected patients being adults (19). There is no previous study on the prevalence of B19 in Iranian SCD patients; however, in the most recent study on 400 Iranian blood donors, Karimnia et. al showed that 60.5% of the samples were positive for anti-B19 IgG antibodies, and found no positive case for IgM antibodies and viral DNA (20). Despite this finding, our research aligns with other global studies indicating that the absence of B19 screening in routine blood bank tests can heighten the risk of B19 transmission. Up to 20% of SCD patients will develop aplastic crisis (21). Among adults with SCD, a staggering 86% of aplastic crisis cases are linked to B19 (22). Our research reveals a significant 90.9% prevalence of B19 in cases of aplastic crisis, providing additional confidence to the existing literature on the subject. Given the established risk posed by B19 in SCD and its causal role in aplastic crises, parallels are postulated with P4, a virus from the same Parvoviridae family.
As delineated in the introduction, P4, a relatively recent member of the Parvoviridae family, has exhibited significantly elevated prevalence among different diseases. There is poor data on the prevalence of this virus in healthy populations and no global or systematic analysis regarding the epidemiological association of this virus in any population. However, human immunodeficiency virus (HIV)-infected blood donors (9) and hemophilia A patients (23) have shown significantly higher susceptibility to P4 infection with 14.4% and 14.47% DNA prevalence, respectively.
To the extent of our knowledge, these are the only studies predating our research to establish a statistically significant correlation between P4 infection and a clinical condition. Notably, both of these studies were conducted in Iran. P4 DNA was also present in 26.5% of plasma pool samples from healthy blood donors in China (24), blood samples of 41% of hepatitis B and 33% of hepatitis C patients in China (25), 3% of French kidney transplant recipients’ blood samples (26), CSF samples of 2 encephalitis cases in India (15), nasal samples of 12.1% of patients with influenza-like syndrome in India (27), and fecal samples of 0.5% of children with gastroenteritis in Ghana (14)
This diversity in transmission routes, particularly the ability of P4 to be transmitted through blood and its resilience, prompted us to investigate SCD as another potential risk factor, which was confirmed by our findings. Despite limited homology with human parvovirus B19, certain key parallels emerge between these familial members. Firstly, the occurrence of a substantial amount of viral DNA indicative of recent infection is uncommon among blood recipients, with viremia being transient and a significant portion of individuals having acquired immunity from previous exposures. Secondly, both viruses are influenced by seasonal and epidemic factors that impact their prevalence. Additionally, both viruses can be found in various tissues; the detection of these viruses in different tissues of healthy individuals might be attributed to the complex pathogenesis of the viruses. Lastly, despite the acute and resolving nature of many parvovirus infections, both P4 and B19 have been demonstrated to persist in blood and other tissues for several years at low levels, even in the presence of specific IgG antibodies, increasing the likelihood of transmission through blood products (28).
While a multitude of suggested risk factors is associated with P4, the pathogenesis of this virus remains poorly understood. This study, pioneering in demonstrating the substantial prevalence of P4 among SCD patients, raises pivotal questions regarding its potential contributions to aplastic crises and other complications, long-term outcomes, the synergistic impact of B19-P4 co-infection, pathogenicity, immunogenicity mechanisms, potential transmission routes in SCD patients, and preventive strategies. Addressing these inquiries necessitates diverse study approaches. Future epidemiological studies should encompass larger populations and rigorous symptom monitoring to yield definitive insights into P4 outcomes.
Since SCD is an inherited medical condition, evaluation of anti-P4 and B19 antibodies in the long term and comparing it with the healthy population could be beneficial in establishing the overall importance of B19 and P4 in these patients. Our time and budget constraints made us opt against such a study and limit this research to a cross-sectional evaluation of the active infection. Tracking and recording symptoms in the predominantly outpatient SCD population is challenging and underscores the importance of concerted efforts in further studies on this subject. However, in the opinion of the authors, overcoming these obstacles is crucial, given the vulnerability and intricacies associated with SCD patients.
5.1. Conclusions
Human Parvovirus 4, a less-explored member of the Parvoviridae family, exhibits certain epidemiological similarities with B19. This study reveals a notable increase in the prevalence of both P4 and B19, as well as their co-occurrence, among individuals with SCD compared to healthy individuals. The prevalence of P4 was found to lack a significant correlation with factors such as age, gender, aplastic crisis, or specific complications in SCD patients.