1. Background
Verrucomicrobia is a collection of Gram-negative, spherical, or rod-shaped bacteria that have been categorized into six monophyletic subdivisions (subphyla, classes) based on 16S rRNA gene library analyses. These microorganisms vary in size across different classes and families, and the guanine + cytosine content ranges from 51 to 67% (1). Verrucomicrobia was suggested as a new division within the bacterial domain by Hedlund et al. (as cited by Rivas-Marín et al.) in 1997 and more recently ranked as a member of the phylum Verrucomicrobiota (2, 3). Members of this large phylum are distributed throughout freshwater, marine, and soil habitats, as well as the digestive tracts of vertebrates, including both humans and animals (4). The type species Akkermansiamuciniphila, discovered in 2004, is a gram-negative, anaerobic, host-derived, mucin-degrading bacterium belonging to the phylum Verrucomicrobia. This bacterium has attracted significant interest in the fields of biological and biomedical research since its discovery (5).
Akkermansiamuciniphila is a member of the gut microbiota and is mostly found in the human intestinal mucosa. This bacterium is present in significant amounts in healthy individuals, plays a crucial role in regulating glucose levels and supporting the immune system in the gut, and has significant importance in the inhibition of some metabolic diseases, such as inflammatory bowel disease (IBD) and obesity (6, 7). The gut is a very complex and dynamic ecosystem, and the habitat of trillions of microorganisms, known as the intestinal microbiota, plays a role in many physiological processes, from digestion and metabolism to the regulation of the immune system (8). In recent years, our understanding of the gut microbiota has increased considerably, revealing its essential role in maintaining the balance of the human body. Bacteria are the dominant population of the gut microbiota (9). The intestinal microbiota provides the body with a wide range of beneficial properties.
Some of the most important functions of these microbes are maintaining the integrity of the mucosal barrier, supplying nutrients such as vitamins, and protecting against pathogens. In addition, the interaction between the commensal microbiota and the mucosal immune system is important for proper immune function (10). The microbial composition of the intestinal microbiota differs in different regions of the digestive tract. The colon has the highest microbial density of any human-associated microbial community studied to date, representing between 300 - 1000 different species (11). Changes in the gut microbiota are associated with the occurrence of metabolic diseases, such as diabetes, obesity, and irritable bowel syndrome; however, there is conflicting information about their role and changes in colorectal cancer (CRC) (12).
2. Objectives
In this study, the frequency of the phylum Verrucomicrobia was investigated in patients with CRC and precancerous gut lesions (PCLs) in comparison to healthy people.
3. Methods
3.1. Sample Collection
Patients who had consulted a gastroenterologist for colonoscopy were examined in the present study. Patients with polyps or masses in the intestine were considered to have CRC or PCL. Additionally, people who did not have evidence of lesions during colonoscopy were considered the control group. Patients who were referred to a gastroenterologist for colonoscopy were examined in the present study. Sampling was performed at the private colonoscopy center and Sayad Shirazi Hospital in Gorgan for one year. Patients with polyps or masses in the intestine were considered to have CRC or PCL.
The inclusion criteria for patients were as follows: Had symptoms of colorectal cancer or had precancerous or cancerous lesions that were recently confirmed by colonoscopy and pathological tests according to the opinion of a highly specialized doctor. The inclusion criteria for the control group were as follows: Admitted to the colonoscopy center of Sayad Shirazi Hospital and Gastroenterology Clinic in the absence of any history of diagnosis of any type of cancer of the gastrointestinal tract, precancerous lesions, or other chronic gastrointestinal diseases based on the above doctor's opinion. Those with a specialty in gastroenterology were considered the control group.
All study participants completed the informed consent form. Intestinal mucosal secretion samples were collected during colonoscopy from 14, 55, and 68 individuals categorized into the CRC, PCL, and control groups, respectively. Mucous secretions were collected from around the cancerous mass or polyp in the CRC and PCL groups, respectively. In the control group, some mucosal secretions were also obtained. A pathologist determined the type of polyp and mass. Mucosal secretion samples were transferred to the microbiology laboratory of Golestan University of Medical Sciences (GoUMS) for microbial analysis. A questionnaire containing demographic information and medical records was completed by all participants.
3.2. DNA Extraction and Polymerase Chain Reaction
DNA extraction from all collected mucosal secretions was performed using a special stool kit (BehGene Company, Iran). The ratio of absorption at wavelengths 260 and 280 was determined using a NanoDrop (Denomie Ds_11 spectrophotometer) to assess the concentration and quality of the prepared DNA. A ratio of ~1.8 was accepted as pure DNA. The extracted DNA samples were then stored at -20°C. The conventional PCR method was used to detect the phylum Verrucomicrobia. 16S rRNA gene-targeted group-specific primers were used. The oligonucleotide sequences of the primers used were 5′-GCCAGCAGCCGCGGTAATACA-3′ and 5′-GCAGACTACAATCTGAACTGGGC-3′ (13). Primer specificity was tested in the NCBI database. Polymerase chain reaction amplification was performed in a Master Cycler gradient instrument (Eppendorf, Hamburg, Germany) under the following thermal conditions: Initial denaturation at 95°C for 5 min; 35 cycles of denaturation at 95°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 45 s; and a final extension at 72°C for 7 min. The PCR products were electrophoresed on a 1.5% agarose gel, visualized by DNA safe stain (SinaClon Bioscience Co., Tehran, Iran), and photographed under UV light. DNA extracted from A. muciniphila, the main species of the A.muciniphila genus, was used as a positive control. It was purchased in the form of oral capsules (Pendulum Life Co., San Francisco, California, USA).
3.3. Statistical Analysis
The chi-square independence test in the statistical package for the social sciences (SPSS) was employed to determine the relationship between the frequency of Verrucomicrobia and the presence of colorectal cancer or polyps. Additionally, discrepancies in clinical characteristics between the two patient groups were assessed. A p-value less than 0.05 indicated statistical significance.
4. Results
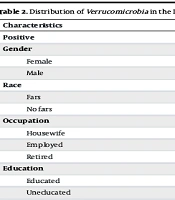
The frequency of Verrucomicrobia in the intestine was significantly greater in healthy individuals than in individuals with cancer or precancerous lesions (75.4% vs. 59.7%, respectively) (P = 0.038). However, there was no significant difference between PCL patients and CRC patients (P > 0.05) (Table 1). Table 2 shows the distribution of Verrucomicrobia in all subjects studied based on demographic data. There was no significant correlation between the presence of Verrucomicrobia and other characteristics, such as age, sex, occupation, residence area (city or village), Body Mass Index (BMI), ethnicity, grade, or family history of colorectal lesions (P > 0.05) (Table 2).
| Verrucomicrobia | Patients | Healthy People | Total | P-Value | |
|---|---|---|---|---|---|
| CRC | PCL | ||||
| Positive | 8 (57.1) | 32 (58.2) | 49 (75.4) | 89 (66.4) | > 0.04 |
| Negative | 6 (42.9) | 23 (41.8) | 19 (24.6) | 48 (33.6) | > 0.05 |
| Total | 14 | 55 | 68 | 137 | > 0.05 |
Abbreviations: CRC, colorectal cancer; PCL, precancerous gut lesions.
a Values are expressed as No. (%).
| Characteristics | Verrucomicrobia | P-Value | |
|---|---|---|---|
| Positive | Negative | ||
| Gender | 0.1 | ||
| Female | 23 (29.8) | 52 (18.54) | |
| Male | 13 (19.2) | 49 (25) | |
| Race | 0.5 | ||
| Fars | 80 (67.2) | 39 (38.8) | |
| No fars | 12 (66.7) | 6 (33.3) | |
| Occupation | 0.5 | ||
| Housewife | 39 (73.6) | 14 (26.4) | |
| Employed | 35 (61.4) | 22 (38.6) | |
| Retired | 18 (66.7) | 9 (33.3) | |
| Education | 0.2 | ||
| Educated | 44 (63.8) | 25 (36.2) | |
| Uneducated | 48 (70.6) | 20 (29.4) | |
| Residency | 0.4 | ||
| Urban | 72 (66.1) | 37 (33.9) | |
| Rural | 20 (71.4) | 8 (28.6) | |
| Family history of colorectal lesions | 0.4 | ||
| Positive | 31 (68.9) | 61 (31.1) | |
| Negative | 14 (66.3) | 31 (33.7) | |
| Mean age | 55.2 ± 12.4 | 57.8 ± 10.5 | 0.2 |
| BMI | 27.3 ± 4.7 | 26.9 ± 4.2 | 0.6 |
a Values are expressed as the mean ± SD or No. (%).
Some people studied were visited by our gastrointestinal subspecialist due to their clinical symptoms, but most of them visited for regular checkups. Our data showed that the frequency of Verrucomicrobia was greater in the intestinal secretions of individuals who underwent regular follow-ups; however, this difference was not significant (P = 0.07) (Table 3). In addition, a significant inverse correlation was observed between Verrucomicrobia and both chronic constipation and anemia in the patient and healthy groups (P = 0.048 and P = 0.001, respectively). However, no significant difference was found between the presence of diarrhea or bloody stool and the frequency of Verrucomicrobia (P = 0.6 and P = 0.2, respectively). There was also no significant association between the frequency of Verrucomicrobia in the intestinal mucosa and a history of diabetes, a history of gastrointestinal diseases in the last two years, blood pressure, vitamin D levels, or a family history of cancer (P > 0.05).
| Characteristics | Verrucomicrobia | P-Value | |
|---|---|---|---|
| Positive | Negative | ||
| The reason for visit | 0.07 | ||
| Check up | 33 (76.7) | 10 (23.3) | |
| Patient | 59 (62.8) | 35 (37.2) | |
| Anemia | 0.048 | ||
| Positive | 25 (55.8) | 67 (44.2) | |
| Negative | 19 (72.0) | 26 (28.0) | |
| Chronic constipation | 0.001 | ||
| Positive | 24 (50.0) | 68 (50.0) | |
| Negative | 24 (77.3) | 21 (22.7) | |
| The presence of diarrhea | 0.6 | ||
| Positive | 11 (68.8) | 81 (31.2) | |
| Negative | 6 (67.5) | 39 (32.5) | |
| Blood in the stool | 0.2 | ||
| Positive | 21 (75.0) | 71 (25.0) | |
| Negative | 7 (65.1) | 38 (34.9) | |
a Values are expressed as No. (%).
5. Discussion
Changes in colorectal cancer is one of the most common types of cancer worldwide. It is the third leading cause of death globally, and its incidence is increasing in developing countries (14). Several factors play a role in CRC development, including environmental factors (15), and changes in the gut microbiome. Studies have shown that changes in the intestinal ecosystem and microbiota are associated with the development of colon cancer or polyps. The phylum Verrucomicrobia is an important group of bacteria known as the natural intestinal microbiota (16).
In 2012, Dubourg et al. investigated the impact of broad-spectrum antibiotics on the gut microbiota of two patients. A significant increase in the abundance of Verrucomicrobia, specifically A. muciniphila, in the gut was detected following antibiotic treatment. This colonization occurred without causing significant gastrointestinal disorders, suggesting that antibiotics can dramatically alter the gut microbiota composition. This study highlighted the complexity and adaptability of the gut microbiota in response to external factors such as antibiotics (17).
Additionally, in a 2017 study, Muriel Derrien et al. highlighted that Akkermansia spp., which are common in the human gut, are linked to metabolic health. An increase in their population, often due to diet or drug changes, improves metabolic parameters, and live A. muciniphila can even protect mice from diet-induced obesity (18). Our research showed that the frequency of Verrucomicrobia in the intestines of patients with precancerous (polyps) and cancerous (tumors) lesions was significantly lower than that in the intestines of healthy people (P > 0.05). A decrease in the abundance of these bacteria may be the basis for the initiation of intestinal changes. Although there are few reports about changes in the frequency of Verrucomicrobia in CRC patients, such changes have been found by several studies for the type species A. muciniphila (19, 20). Wang et al. showed that A.muciniphila was significantly reduced in patients with signs and symptoms of IBD and in mice with colitis or CRC (21).
Some researchers have suggested that the microbiota plays a crucial role in the early stages of intestinal changes leading to polyp formation. However, these bacteria are replaced over time, and their frequency increases (16). This study also revealed no considerable difference in the rate of Verrucomicrobia abundance between intestinal polyps and cancer, indicating that even after a tumor develops, there are no favorable conditions for the growth of these bacteria.
The subjects who participated in the present study were divided into two groups. The first group consisted of individuals who did not exhibit any digestive problems and underwent regular checkups. The frequency of Verrucomicrobia in this group was 76.7%. The second group consisted of individuals who either had digestive symptoms or were suspected to have cancer, so they were recommended for colonoscopy. Some of the second group were found to have polyps or tumors, whereas others had no lesions and were included in the healthy control group. The frequency of Verrucomicrobia in the patient group was 62.8%.
Therefore, it is possible that other gastrointestinal and IBDs that were not examined in this study are related to the reduction or elimination of Verrucomicrobia in colorectal mucus. The results of this study demonstrated a relationship between the presence of Verrucomicrobia in the intestinal mucosa and chronic constipation. The prevalence of these bacteria in patients with chronic constipation was significantly lower than in other patients. However, no association was observed between diarrhea and Verrucomicrobia. It is important to note that changes in bowel habits are the most common symptom of colon cancer: There could be an increase in constipation or alternating periods of constipation and diarrhea. Prolonged elimination may lead to prolonged exposure of the colonic mucosa to possible carcinogens present in feces. However, there are conflicting results from previous studies regarding the association between chronic constipation and the risk of colorectal cancer (22).
Colon cancer can be accompanied by iron deficiency as an early symptom of the disease. Patients with CRC frequently experience iron deficiency anemia and require iron therapy (23). The abundance of gut Verrucomicrobia was significantly correlated with severe anemia in our study, supporting previous findings. Slow bleeding caused by large colon tumors can be detected through occult blood (OB) tests or stool examinations and can serve as a diagnostic marker over time. However, our study did not find any association between the absence or presence of blood in feces and the abundance of Verrucomicrobia. The rarity of advanced and large tumors in the present study could explain this finding. According to the World Health Organization (WHO), excessive fat accumulation, overweight status, and obesity (BMI) are significantly associated with an increased risk of CRC (24), but other studies have not shown such relationships (25). Our study also revealed no significant relationship between the BMI of patients and the presence of Verrucomicrobia (Table 2).
5.1. Conclusions
This study suggested that Verrucomicrobia, a type of bacteria found more frequently in healthy individuals, may play a crucial role in maintaining human health and could protect against CRC, intestinal polyps, chronic constipation, and anemia. However, further research is needed to confirm these findings and understand the exact nature of the role of Verrucomicrobia under these conditions. This could lead to new strategies for preventing and treating a range of health conditions.