1. Background
Alcaligenaceae, which are included in the order Burkholderiales, are a family of bacteria. Alcaligenes faecalis is a Gram-negative, obligate, nonfermenting aerobe that is motile with peritrichous flagella and a non-pigmented rod (1). Alcaligenes faecalis has been linked to a variety of human clinical infections. Sixty-two A. faecalis infections were sporadically reported in the medical literature before 1997 (2). Meningitis and bacteremia were the most frequently reported cases, and the majority of these infections were reported in newborns and infants. Most patients were treated with sulfonamides. In 1960, Doxiadis reported 33 cases of bacteremia in newborns, which was the largest case series of A. faecalis bacteremia. Alcaligenes faecalis is resistant to sulfonamides, and A. faecalis bacteraemia caused 20 fatalities (3).
There were 127 sporadically reported cases of A. faecalis infection in the literature after 1997 (2). In Angola, Fillipe documented 20 cases of chronic otitis media (4). These instances of A. faecalis chronic otitis media were associated with the locals' use of bird feces as a traditional therapy to avoid ear discharge. The cases reported in the literature after 1997 (excluding the 20 cases of chronic otitis media in Angola) indicate that urinary tract infections, skin and soft tissue infections, and pulmonary infections were the most common locations of A. faecalis infection (2). Here, we report a case series of patients with A. faecalis pneumonia.
2. Objectives
The aim of this study was to elucidate the clinical characteristics, management strategies, and outcomes of A. faecalis pneumonia patients.
3. Methods
We retrospectively analyzed all patients who were hospitalized at Dalin Tzu Chi Hospital between January 2014 and December 2019 with pneumonia caused by A. faecalis. The trial was open to any adult patient who met all the requirements and had a confirmed radiological and clinical diagnosis of pneumonia. The criteria used to define pneumonia were as follows: (1) chest radiography showing a new or developing infiltrate (s); (2) fever (defined as an oral temperature greater than 38 °C); and (3) purulent sputum or a change in the properties of sputum; (4) a new or worsening cough; (5) dyspnoea or tachypnea; (6) audible results, such as rales and/or signs of lung consolidation, upon pulmonary examination; (7) a peripheral white blood cell count of more than 10,000 cells/mm3.
Patients were diagnosed with pneumonia when they had clinical symptoms and signs of pneumonia, and their chest radiography revealed new pulmonary infiltration. Bronchial lavage fluid or respiratory secretions were obtained for culturing. Through the use of the VITEK II system and ASTGN87 cards (BioMérieux, Marcy-l'Étoile, France) with the Clinical and Laboratory Standards Institute interpretation criteria M100 - 25th, bacterial cultures of respiratory secretions and tests for antibiotic resistance were conducted. As soon as respiratory secretions were obtained, they were cultured on colistin-nalidixic acid agar, blood agar, chocolate agar, and Eosin Methylene Blue Agar. The transfer tube and matching suspension tube were placed in the adjacent slot along with one GN identification card (automated identification of 135 taxa of the most significant fermenting and nonfermenting Gram-negative bacilli) and another card of VITEK II AST-N322 (for susceptibility testing of aerobic Gram-negative bacilli against specific antimicrobials).
The results of every test were evaluated using the raw data and compared to thresholds. We procured patient data and medical records through the use of a microbiology laboratory reporting system. The data collected encompassed a spectrum of aspects, including demographics, clinical symptoms, and signs, imaging findings, risk factors for pneumonia, previous intravenous antibiotic use within 90 days, pulmonary secretion culture results, antibiotic sensitivity test results, and, ultimately, clinical outcomes. The definition of extensively drug-resistant/pan-drug-resistant cites the study of Maggiorakos et al. (5).
4. Results
4.1. Case Report
An 82-year-old male patient has been hospitalized at Dalin Tzu Chi Hospital five times due to pneumonia since December 2017. He received a tracheostomy on December 20, 2017. He experienced productive cough and dyspnoea on February 16, 2019. He visited our emergency room for help. His chest radiograph showed a chronic fibrotic lesion over both lungs with waving of both diaphragms and alveolar opacity over the left upper lung. At the time of pneumonia diagnosis, he was admitted to our ward for further management. His sputum culture on February 17, 2019, revealed A. faecalis growth. We thought that A. faecalis was a contaminating bacterium.
The patient was treated with cefpirome (1.0 gram) intravenously every 12 hours. His condition improved gradually. He suffered from fever again on February 23, 2019, and his chest radiograph showed alveolar opacity in the left lung. His sputum culture on February 24, 2019, revealed the presence of A. faecalis, Pseudomonas aeruginosa, and Escherichia coli. We intravenously administered cefoperazone-sulbactam (2.0 grams) for 12 hours to the patient. After seven days of intravenous cefoperazone-sulbactam therapy, he experienced fever improvement in the ward. Unfortunately, he suffered from choking, fever, and dyspnoea on March 1, 2019. His chest radiograph on March 1, 2019, revealed progressive increasing alveolar opacity over the left lung. His sputum culture showed A. faecalis growth.
We determined that A. faecalis was a pathogen, not a contaminating bacterium. A. faecalis is only sensitive to tigecycline and resistant to many antibiotics, such as anti-pseudomonas penicillin, cephalosporins, carbapenems, quinolones, and aminoglycosides. Therefore, we prescribed 50 milligrams of intravenous tigecycline every 12 hours. After treatment, the severity of A. faecalis pneumonia was well controlled. His chest radiograph on March 18, 2019, revealed a resolution of the left lung alveolar opacity. He was discharged on March 18, 2019. Table 1 shows the complete course of the patient's hospitalization.
| Hospital Day | Temporal Temperature (°C) | WBC Count | CRP mg/dL | Sputum Culture | Antibiotics | Comment |
|---|---|---|---|---|---|---|
| 1 | 36.8 - 37.4 | 7200 | 0.99 | Cefpirome | Admisson | |
| 2 | 36.5 - 38.0 | Alcaligenes faecalis | Cefpirome | Fever | ||
| 3 | 36.0 - 38.3 | Alcaligenes faecalis | Cefpirome | Fever | ||
| 4 | 36.1 - 37.0 | Cefpirome | ||||
| 5 | 36.2 - 37.0 | Cefpirome | ||||
| 6 | 36.0 - 37.1 | Cefpirome | ||||
| 7 | 36.0 - 37.2 | Cefpirome | ||||
| 8 | 37.2 - 38.5 | 10260 | 6.09 | Alcaligenes facealis | CPZ-sulbactam | Fever |
| 9 | 36.1 - 37.8 | Pseudomonas aeruginosa and Escherichia coli | CPZ-sulbactam | |||
| 10 | 36.0 - 36.8 | CPZ-sulbactam | ||||
| 11 | 36.1 - 36.9 | CPZ-sulbactam | ||||
| 12 | 36.3 - 36.9 | CPZ-sulbactam | ||||
| 13 | 36.0- 37.0 | CPZ-sulbactam | ||||
| 14 | 36.4 - 38.8 | CPZ-sulbactam | Fever | |||
| 15 | 36.0 - 38.8 | CPZ-sulbactam | Fever | |||
| 16 | 36.8 - 38.5 | 11830 | 10.2 | Alcaligenes faecalis | Tigecycline | Fever |
| 17 | 36.8 - 38.6 | Tigecycline | Fever | |||
| 18 | 36.3 - 38.3 | Tigecycline | Fever | |||
| 19 | 36.7 - 38.1 | Tigecycline | Fever | |||
| 20 | 36.6 - 37.8 | Tigecycline | Fever | |||
| 21 | 36.0 - 37.1 | Tigecycline | ||||
| 22 | 36.0 - 36.9 | Tigecycline | ||||
| 23 | 36.0 - 36.2 | Tigecycline | ||||
| 24 | 36.0 - 36.9 | Tigecycline | ||||
| 25 | 36.0 - 36.0 | Tigecycline | ||||
| 26 | 36.0 - 36.6 | Tigecycline | ||||
| 27 | 36.0 - 36.8 | Tigecycline | ||||
| 28 | 36.1 - 36.9 | Tigecycline | ||||
| 29 | 36.0 - 36.6 | Tigecycline | ||||
| 30 | 36.0 - 36.1 | Tigecycline | ||||
| 31 | 36.0 - 36.3 | Tigecycline | Discharge |
Whole Complete Course of Patient’s Hospitalization
4.2. Case Series
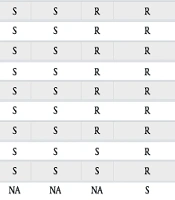
All patients who were hospitalized with A. faecalis pneumonia at Dalin Tzu Chi Hospital from January 2014 to December 2019 were retrospectively analyzed. Eight patients with A. faecalis pneumonia were diagnosed, including six males and two females. The age distribution ranged from 51 to 83 years. The mean age was 70.0 years. The following patient conditions increased their likelihood of pneumonia: Four bedridden patients with cognitive impairments, three with underlying cancers, and one with end-stage renal disease. Seven patients had a history of prior intravenous antibiotic use within 90 days. Sputum cultures from six patients revealed polymicrobial infections. Antibiotic sensitivity tests revealed the emergence of extensively drug-resistant A. faecalis in 2018. A patient with stage IV adenocarcinoma of the lung receiving chemotherapy did not receive adequate intravenous antibiotic therapy and died due to A. faecalis pneumonia. Another patient with stage IIIA squamous cell carcinoma of the lung receiving sequential radiotherapy and chemotherapy received adequate intravenous antibiotic therapy but still died due to A. faecalis pneumonia. After receiving sufficient intravenous antibiotic medication, six patients were released with perfect health. The bacteriology and clinical outcomes of the eight patients are displayed in Table 2 (2).
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case7 | Case8 |
|---|---|---|---|---|---|---|---|---|
| Year | 2014 | 2014 | 2015 | 2018 | 2019 | 2019 | 2019 | 2019 |
| Prior intravenous antibiotic use within 90 days | No | ETP FEP VAN | LVX | CAZ LVX DOR VAN | CAZ AMK IPM TEC CIP | TZP MEM CAZ TEC AMK | CRO SAM | TZP MEM CAZ VAN LVX AMK |
| Antibiotics therapy | CXM | CRO | FEP | TGC | TGC | MEM | CAZ | CAZ |
| Outcome | Cure | Cure | Dead | Cure | Cure | Cure | Dead | Cure |
| Results of antibiotics sensitivity test of Alcaligenes faecalis | ||||||||
| GEN | S | S | R | R | R | R | S | NIL |
| AMK | S | S | R | R | R | R | S | NIL |
| CAZ | S | S | R | R | R | S | S | NIL |
| FEP | S | S | R | R | R | R | S | NIL |
| SAM | S | S | R | R | R | R | S | NIL |
| TZP | S | S | R | R | R | S | R | NIL |
| CIP | S | S | R | R | R | R | R | NIL |
| IPM | S | S | S | R | R | S | S | NIL |
| MEM | S | S | S | R | R | S | S | NIL |
| TGC | NA | NA | NA | S | S | NA | NA | NIL |
| Mixed infection pathogens | ||||||||
| Proteus vulgaris | V | |||||||
| MSSA | V | |||||||
| Pseudomonas aeruginosa | V | V | ||||||
| Providencia stuartii | V | |||||||
| Brukholderia cepacia | V |
Bacteriology and Clinical Outcome in Eight Cases of Alcaligenesfaecalis Pneumonia a
5. Discussion
In 1983, Gray et al. reported a pathogenetic change in the trachea of turkey infected with A. faecalis (6). The pathogenesis of A. faecalis pneumonia in humans is unknown. No authors have explored this issue or reported the findings in the literature. Very few case reports of A. faecalis pneumonia exist. In 2006, Leesik et al. reported on microbial pathogens in adults with community-acquired pneumonia, including two cases of community-acquired pneumonia caused by A. faecalis (7). Ergul et al. reported two cases of A. faecalis ventilator-associated pneumonia in a pediatric intensive care unit in 2017 (8). However, these two articles included no description of the clinical information about A. faecalis pneumonia patients. Agarwal et al. reported a 32-year-old male patient who had hemorrhagic fever caused by dengue in 2017. His chest radiograph revealed alveolar opacities in both lungs (9).
Using bronchoalveolar lavage fluid culture, A. faecalis was isolated. The results of the antibiotic sensitivity test indicated sensitivity to tigecycline and colistin and resistance to carbapenems, quinolones, aminoglycosides, cephalosporins, monobactam, minocycline, and ureidopenicillins. This patient later succumbed to his illness (9). According to a 2018 study by Junejo et al., a 73-year-old man had bilateral alveolar opacities on his chest radiograph. Culture and sensitivity analysis revealed that the sputum-isolated A. faecalis strain was resistant to anti-Pseudomonas penicillins, carbapenems, aminoglycosides, and quinolones. After receiving polymyxin B treatment, the patient's hemodynamic stability improved (10). Al-Zakhari et al. reported the death of a 66-year-old male patient in 2020. The patient suffered from cavitary pneumonia caused by pan-drug-resistant A. faecalis. The patient died despite aggressive antibiotic treatment (linezolid and polymyxin B) (11). Patients 4 and 5 had extensively drug-resistant A. faecalis pneumonia in this study, and the results for antibiotic sensitivity were the same as those in Agarwal and Junejo's reports (9, 10).
In Bizet's study, A. faecalis containing a β-lactamase was discovered (1). In Pereira’s study, a strain of A. faecalis resistant to expanded-spectrum beta-lactamase cephalosporins was identified in the urine of a patient (12). Agarwal et al. reported a case of a patient with extensively drug-resistant A. faecalis pneumonia (9). Hasan et al. reported a patient with pan-drug-resistant A. faecalis bacteraemia who received double-dose tigecycline and had a successful treatment outcome (13). In 2018, a strain of extensively drug-resistant A. faecalis susceptible to only tigecycline was isolated in our hospital.
Among our two patients with extensively drug-resistant A. faecalis pneumonia, both had a history of prior intravenous antibiotic use within 90 days, which may be a risk factor for extensive drug-resistant A. faecalis infection. Of the seven patients with A. faecalis pneumonia reported in the literature and our eight patients, four died (26.7%). The other eleven patients received adequate intravenous antibiotic therapy and recovered. The pathogenicity of A. faecalis is minimal. Patients with A. faecalis infection usually have a good prognosis with appropriate intravenous antibiotic therapy. We emphasize the fact that life-threatening A. faecalis infections have been reported.
5.1. Conclusions
Recently, extensively drug-resistant A. faecalis strains have emerged. The use of polymyxin B or tigecycline is necessary for patients with extensively drug-resistant A. faecalis pneumonia. Patients with A. faecalis pneumonia usually have a good prognosis after receiving appropriate intravenous antibiotic therapy. However, fatal cases of pneumonia have been reported in the literature.