1. Background
Invasive aspergillosis (IA) is a major cause of morbidity and mortality in patients with hematological malignancies (1-3). Aspergillus species are prevalent filamentous etiological agents. While the respiratory system (lungs and sinuses) is the most common site of infection, other organs, such as the central nervous and cardiovascular systems, can become infected due to hematogenous dissemination (1, 2, 4, 5). In recent years, the rate of invasive aspergillosis has increased due to a rise in the number of immunocompromised patients (6). Invasive aspergillosis is the most common fungal infection among stem cell and solid organ transplant recipients (7, 8).
Invasive aspergillosis ranks among the four diseases with the highest mortality rates in the US healthcare network. The incidence rate of IA increased by 0.3 per year between 2000 and 2013 (6). According to Jacobs et al., the mortality rates of invasive respiratory aspergillosis in hematologic patients with and without voriconazole (VOR) therapies were 48.6% and 100%, respectively (9). The ongoing increase in IA incidence is attributed to the trend toward more aggressive chemotherapeutic regimens and extensive use of immunosuppressive agents. However, more successful outcomes are being achieved in IA therapy due to advancements in antifungal strategies and therapies (10).
Voriconazole, a triazole antifungal agent, is used in high-risk patients with serious fungal infections, including those caused by resistant yeasts and filamentous fungi (11, 12). The updated consensus guidelines (13) for diagnosing and managing IA recommend VOR therapy incorporating therapeutic drug monitoring (TDM) as a primary treatment. Voriconazole therapy was initiated according to the literature with 6 mg/kg of the patient's weight IV (intravenously q12hr) for the first 24 hours, followed by 4 mg/kg IV q12hr (13). Additionally, early initiation of antifungal treatment for IA is crucial to prevent mortality, and treatment should not be delayed while awaiting mycological test results (13). Therapeutic drug monitoring of VOR is a valuable tool for optimizing VOR plasma concentration and minimizing toxicity. Many factors affect the blood concentration of VOR, such as age, drug-drug interactions, and genetic polymorphisms of cytochrome CYP2C19 (14, 15).
The metabolism of numerous therapeutic agents is controlled by Cytochrome P450 2C19 (CYP2C19). Highly polymorphic genes of this enzyme influence the metabolism of therapeutic drugs (16). The functional allele form of CYP2C19 is CYP2C19*1 (wild-type) (17). Other CYP2C19 polymorphic alleles with single-base substitutions include *2 (rs4244285, 681G > A), *3 (rs4986893, 636G > A), and *17 (rs12248560, 806C > T). The CYP2C19*1*1 wild-type phenotype is an extensive metabolizer with normal enzyme activity. Two polymorphic alleles (*2 and *3) reduce the ability to metabolize drugs (18). The CYP2C19*17 gene variant results in ultra-rapid metabolism (URM) of CYP2C19 substrates (19).
The CYP2C19*2*2, *3*3, and *2*3 are known as poor metabolizers (PM). CYP2C19*1/*2, *1/*3, *1/*17, and *2/*17, with intermediate activity, are heterozygote extensive metabolizers (HEM) (18, 19)). Various adverse effects are associated with VOR concentration, such as visual disturbances, liver enzyme elevation, visual hallucinations, skin rash, and psychiatric symptoms, as defined by the Common Terminology Criteria for Adverse Events (CTCAE) by the National Cancer Institute (20). To optimize the therapeutic efficacy of VOR, plasma concentrations of VOR and CYP substrates (cyclosporine, tacrolimus, and sirolimus) should be considered (21). Several studies reported the efficacy and safety of VOR therapy with TDM in pediatrics (22, 23)). However, few studies have focused on TDM of VOR in pediatric patients with hematologic disorders.
2. Objectives
This study aims to determine the VOR plasma level and related factors such as age, sex, liver function, C-reactive protein (CRP) level, white blood cell (WBC) count, and erythrocyte sedimentation rate (ESR) in pediatric patients with hematologic disorders. A detailed investigation of the CYP2C19 genotype will be conducted to explore the association between VOR concentration and the CYP2C19 genotype in these patients.
3. Methods
The inclusion criteria consisted of hematologic patients with IA treated at two university hospitals (Nemazi and Amir) in Shiraz, Iran, who received VOR for prophylaxis or treatment. Patients were excluded if they had been admitted to the ward and received VOR treatment for more than 3 days. The diagnosis of IA was confirmed based on signs and symptoms of infections depending on the site of infection, including pleural or back pain or dyspnea indicating lung involvement, headache, altered mental state, seizure, or focal neurologic signs confirming central nervous system involvement (24). Patients were classified according to their signs and symptoms, following the criteria outlined by the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (25).
In our study, the diagnosis of IA in patients relied on symptoms and tests reported previously (24). Invasive aspergillosis in high-risk patients suffering from clinical signs and symptoms was confirmed with positive results for KOH smear and fungal culture in clinical samples like sputum, bronchoalveolar lavage (BAL) fluid, and tissue (lung or sinuses) biopsies. Results of pathology smear showing branching mycelium, the presence of radiologic abnormalities such as a halo and air-crescent sign, cavitation, nodule, or a mass near a large vessel in the lung, and positive results of PCR evaluations in clinical samples (26). Treatments were documented by clinicians based on clinical, radiological, and mycological findings. VOR doses administered to pediatric patients were prescribed by clinicians according to their age and weight (27).
To avoid contradiction with chemotherapy medications, VOR administration started with a low dosage, and then the adjustments were made according to VOR concentration level measurements in the related patients. The response to VOR therapy in patients was evaluated by assessing the degree of recovery in signs and symptoms, negative results of laboratory diagnostic methods, changes in computerized tomography (CT) or magnetic resonance imaging (MRI) results, and progression of infection or death (21). Successful treatment was defined as either complete treatment or partial treatment (treatment progress accompanied by mild signs and symptoms, with continued treatment). Adverse drug reactions, concomitant medications (i.e., diazepam, midazolam, tacrolimus, sirolimus, cyclosporine, warfarin, efavirenz, rifamycin, phenytoin, ritonavir, omeprazole, pantoprazole, or prednisolone), results of liver function tests, WBC count, CRP and ESR levels, and patient outcomes were collected from the electronic medical record information system of the hospitals.
Blood samples (3 mL) were collected from the patients 30 min before the next VOR dose on the 3rd, 5th, and 7th days after receiving VOR. The samples were centrifuged at 3000 rpm for 10 min. Voriconazole concentrations were evaluated using reverse-phase high-performance liquid chromatography (HPLC) (4). For protein precipitation, 200 µL of plasma and 200 µL of cold acetonitrile were vortexed for 30 s and centrifuged at 13800 rpm for 15 min. After centrifugation, the supernatants were filtered through a 0.22 µm pore syringe filter. A Knauer analytical HPLC (Berlin, Germany) equipped with a Nucleodur 100-5 C18 ec 125 × 4.6 mm column and K-1001 pump was used. A variable-wavelength ultraviolet (UV) spectrophotometer was used as the detector with EZChrom Elite software utilized for analysis. A mixture of deionized water and acetonitrile (≥ 99.9%, HPLC grade, Merck, Germany) in a ratio of 60/40 (V/V) was used as the mobile phase. A flow rate of 0.4 mL/min and a detection wavelength of 262 nm were applied. The injection loop and total run time were 20 μL and 15 min, respectively. The standard stock solution included 1.63 mg of VOR powder (≥ 98% HPLC grade, Sigma-Aldrich) in 1 mL dimethyl sulfoxide (≥ 99.9%, Merck, Germany). Solutions of different concentrations were prepared using human male AB serum (Sigma-Aldrich) as the solvent.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used for genotyping to investigate whether a significant association exists between VOR concentration and the CYP2C19 genotype in patients. Genomic DNA was extracted using the Sinacolon extraction kit (Tehran, Iran). The forward and reverse oligonucleotide primers used for amplifying the alleles included forward CYP2C19*2 primer: 5′-AATTACAACCAGAGCTTGGC-3′, reverse CYP2C19*2 primer: 5′-TATCACTTTCCATAAAAGCAAG-3′; forward CYP2C19*3 primer: 5′-AACATCAGGATTGTAAGCAC-3′, reverse CYP2C19*3 primer: 5′-TCAGGGCTTGGTCAATATAG-3′, forward CYP2C19*17 primer: 5′-GCCCTTAGCACCAAATTCTC-3′, reverse CYP2C19*17 primer: 5′-ATTTAACCCCCTAAAAAAACACG-3′ (16). The reaction mixture for PCR amplification consisted of 2 µL genomic DNA, 1 µL (1 pM) of each primer, and 8.5 µL double distilled water. The process was performed as follows: Initial denaturation at 95°C for 5 min, followed by 34 cycles of 95°C for 45 s, 60°C for 45 s, 72°C for 45 s, and a final extension of 7 min at 72°C. The PCR products were electrophoresed on a 1.5% agarose gel (Powerpack, Bio-Rad) at 70 V for 60 min. The 168-bp, 119-bp, and 473-bp amplified fragments for the *2, *3, and *17 alleles were presented, respectively.
The SmaI, BamHI, or LweI (Thermo Fisher Scientific, Inc.) restriction endonucleases were used for CYP2C19*2, CYP2C19*3, and CYP2C19*17 restriction digestion, respectively. For PCR product digestion, 5 µL PCR products, 9 µL double distilled water, 0.5 µL of SmaI, BamHI, or LweI enzyme, and 1 µL of their 10X buffer were used and incubated at 30°C, 37°C, and 37°C, respectively, for 16 h (overnight). They were then inactivated at 65°C, 80°C, and 65°C for 20 min. The digested PCR products were separated using 2.5% agarose gel electrophoresis at 77 V for 50 min. Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). Categorical variables were compared using the χ2 test or ANOVA variance. Spearman's rho correlations and multivariate analysis by linear regression were applied to analyze the effective factors on VOR trough concentrations. Statistical significance was defined as a two-sided P-value of < 0.05.
4. Results
Forty-four patients with hematologic disease (including Brockett lymphoma, acute myeloid leukemia, malignant neoplasm lymphoma, acute lymphoblastic leukemia, thalassemia, chronic granulomatous disease, aplastic anemia, pancytopenia, and non-Hodgkin lymphoma) were included in this study. The mean and median ages of the patients were 6.95 years and 6 years, respectively. The female-to-male ratio was 17/27 (38.6%/61.4%). The most frequent underlying diseases were acute lymphoblastic leukemia (29.5%, 13/44) and acute myeloid leukemia (20.5%, 9/44). The site of infection was the lungs in 65.9% (29/44) of patients. Other infection sites included the sinuses, liver, brain abscesses, and skin. During the study period, 65.9% (29/44) and 11.4% (5/44) of patients were treated completely and partially, respectively, and 22.7% (10/44) died. IA in the patients was classified as 20.5% proven, 77.2% probable, and 2.3% possible.
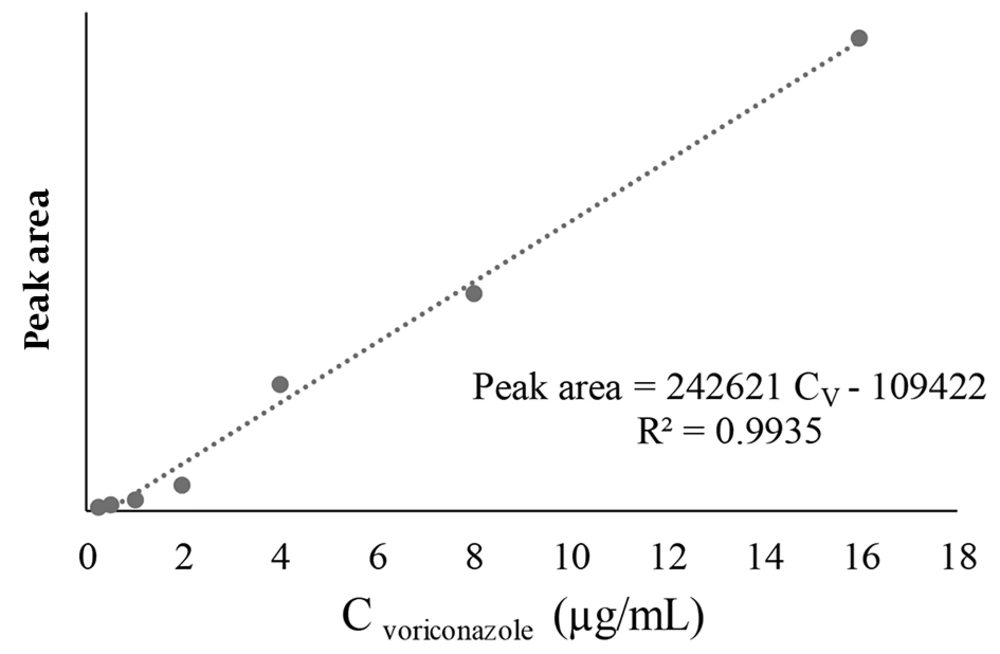
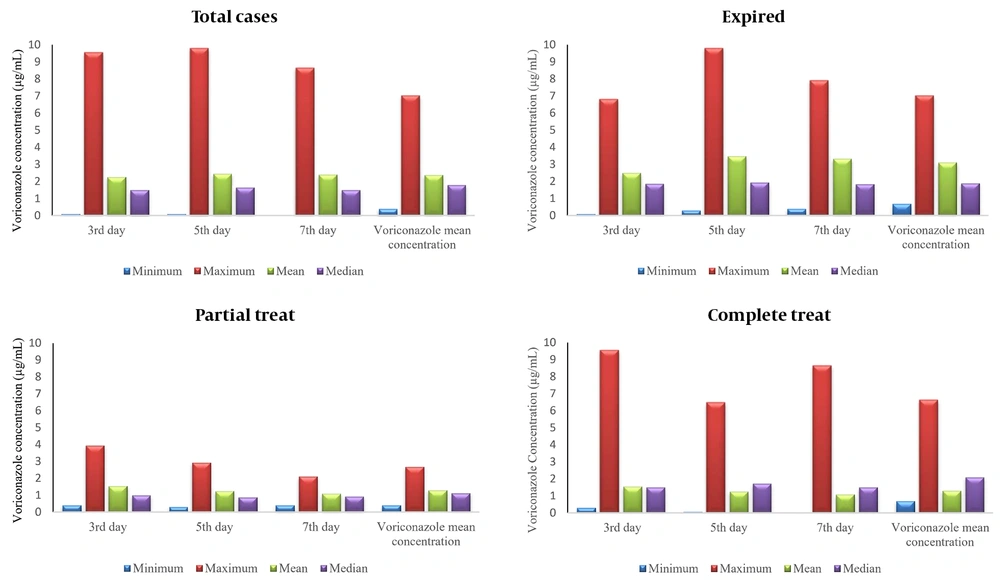
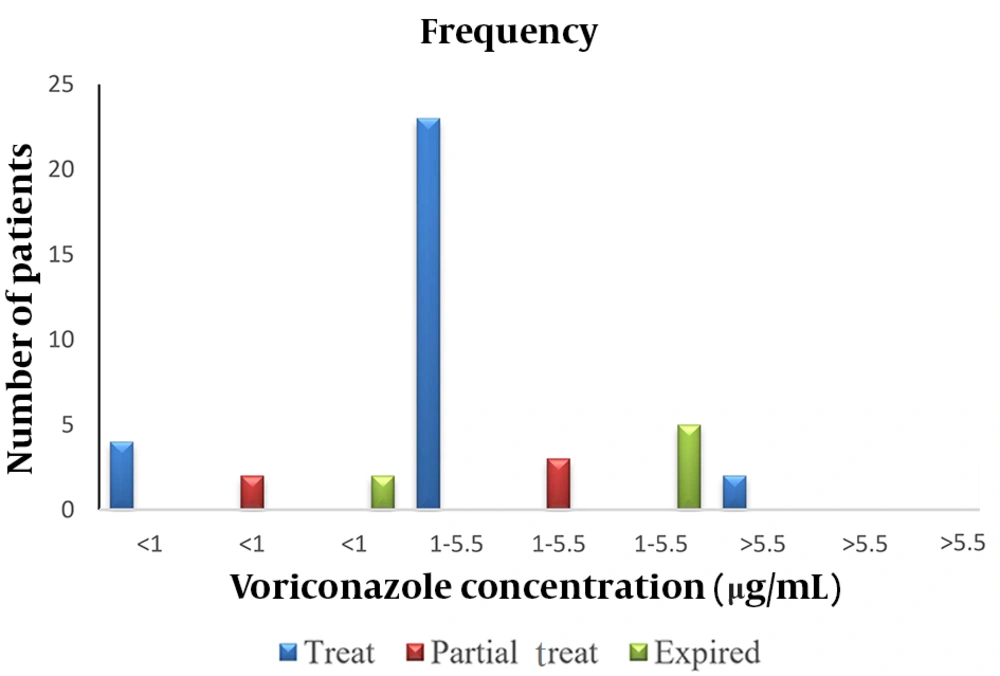
The peak areas of the VOR levels versus the concentration of the standard solution were plotted on three different days for the calibration curve (Figure 1). The peak areas derived from the HPLC chromatogram were used as the signal intensity related to the concentration of standard VOR solutions. A linear relationship was observed between the peak area and the analytical concentration of VOR. The calibration curve was used to calculate the concentration of VOR in patient samples. Voriconazole concentrations were evaluated in 132 samples from 44 pediatric patients. The means of plasma VOR concentrations on the 3rd, 5th, and 7th days of VOR administration were 2.27 µg/mL (range 0.10 - 9.56 µg/mL), 2.44 µg/mL (range 0.09 - 9.80 µg/mL), and 2.39 µg/mL (range 0.007–8.66 µg/mL), respectively (Figure 2). The VOR concentration range in 77.3% (34/44) of treated patients (complete and partial) was within 1-5.5 µg/mL (Figure 3). The number of patients with VOR concentrations less than 1 µg/mL and more than 5.5 µg/mL was 18.2% (8/44) and 11.4% (5/44), respectively.
Patient demographic data and clinically effective factors such as age, WBC count, CRP, ESR, and liver function markers are presented in Table 1. Sex was not a significant factor, but age had a significant effect on the 3rd-day blood VOR concentration (P = 0.013). Multivariate analysis by linear regression showed that every 1-year increase in age increased VOR concentration by 0.037 µg/mL. The mean CRP level and ESR in the studied patients were 47.40 mg/L and 63.68 mm/h, respectively (Table 1). C-reactive protein significantly influenced the concentrations of VOR on the 3rd day of administration (P = 0.022). The enhancement of this factor by one unit would increase the mean VOR concentration by 0.06 µg/mL on the 3rd day. ESR did not have any significant effect on the trough levels of VOR on the 3rd, 5th, and 7th days of VOR administration (P = 0.076, 0.799, and 0.870, respectively).
The mean values of alkaline phosphatase (ALKP), alanine aminotransferase (ALT), aspartate transaminase (AST), direct bilirubin (DBil), total bilirubin (TBil), and albumin (Alb) in pediatric patients were 381.25 u/L, 38.41 u/L, 30.72 u/L, 0.44 mg/dL, 2.46 mg/dL, and 3.92 g%, respectively (Table 1). The evaluation of these factors showed that their values were higher than normal values, except for Alb, but none of these factors had significant effects on concentration levels of VOR, using the Spearman rho correlation test. Multivariate analysis showed a significant effect of ALT level only on the mean VOR concentration (P = 0.032). Increasing ALT by 1 unit decreased the mean VOR concentration by 0.03 µg/mL. The statistical bivariate analysis demonstrated that WBCs were not significantly effective on VOR concentrations on any day (P = 0.397, 0.880, and 0.857).
| Variables | Minimum | Maximum | Mean ± SD | Normal Range | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Voriconazole concentration on 3rd day | 0.10 | 9.56 | 2.27 ± 0.33 | 1 - 5.5 | 1 - 5.5 |
| Voriconazole concentration on 5th day | 0.09 | 9.80 | 2.44 ± 0.32 | 1 - 5.5 | 1 - 5.5 |
| Voriconazole concentration on 7th day | 0.007 | 8.66 | 2.39 ± 0.34 | 1 - 5.5 | 1 - 5.5 |
| Age (y) | 1 < | 14 | 6.95 ± 0.63 | - | - |
| Erythrocyte sedimentation rate (mm/hr) | 3.00 | 125.00 | 63.68 ± 6.76 | 1 - 20 | 1 - 30 |
| C-reactive protein (mg/L) | 2.00 | 150.00 | 47.40 ± 5.05 | 0 - 6 | 0 - 6 |
| Alkaline phosphatase (U/L) | 32.00 | 1657.00 | 381.25 ± 52.44 | 98 - 279 | 80 - 306 |
| Alanine aminotransferase (u/L) | 2.10 | 190.00 | 38.41 ± 6.59 | 0 - 31 | 0 - 31 |
| Aspartate transaminase (u/L) | 2.60 | 76.00 | 30.72 ± 2.91 | 0 - 31 | 0 - 31 |
| Direct bilirubin (mg/dL) | 0.05 | 2.67 | 0.44 ± 0.09 | < 0.3 | < 0.3 |
| Total bilirubin (mg/dL) | 0.18 | 56.00 | 2.46 ± 1.46 | 0.1 - 1.2 | 0.1 - 1.2 |
| Albumin (g%) | 2.20 | 4.80 | 3.92 ± 0.08 | 3.5 - 5 | 3.5 - 5.2 |
| White blood cell (× 1000/mm3) | 0.07 | 39.54 | 6.19 ± 1.30 | 3.5 - 10.5 | 4 - 10 |
The genotypes of 37 patients were analyzed. No patient had the CYP2C19*3 mutation. The frequencies of the wild-type CYP2C19*1 and mutation types of CYP2C19*17 and CYP2C19*2 alleles were 39.2% (29/74), 21.6% (16/74), and 39.2% (29/74), respectively. Among the studied patients, 2 (5.4%) had the CYP2C19*1*1 genotype, 6 (16.2%) CYP2C19*1*17, 19 (51.4%) CYP2C19*1*2, and 10 (27%) CYP2C19*2*17. The prevalence rates of CYP2C19 phenotypes for EM (extensive metabolizer) and HEM (heterozygote extensive metabolizers) were 5.4% (2/37) and 94.6% (35/37), respectively, with no URM (ultra-rapid metabolizer) or PM (poor metabolizer) phenotypes. There was no significant association between VOR concentration and the CYP2C19 genotype in patients. Voriconazole-related adverse effects were observed in 2/44 patients (4.5%). The mean VOR concentration in one patient (CYP2C19*2/*17 genotypes) was more than 5.5 µg/mL; in the other (CYP2C19*1/*2 genotypes), it was within the normal range. The adverse events were skin rash, fever, nausea, and visual disturbance. In the present study, limited patients used concomitant medications; therefore, the effects of such medications on VOR concentrations could not be evaluated. Ten patients (22.73%) died, and no significant correlation was detected between the VOR concentration and death.
5. Discussion
In the present study, the VOR plasma concentrations in patients with hematologic disorders were evaluated. The respective ranges in the most treated cases were 1 - 5.5 µg/mL. No significant relationship was observed between sex and VOR concentration in the present study; however, age was significantly related to VOR concentration (P = 0.013) on the 3rd day. There was no significant relationship between sex (P = 0.48) or age (P = 0.705) and VOR concentrations in a study by Hu et al. (28) in pediatric patients. The same results were reported in a study on liver transplant recipients treated with VOR (P = 0.618 for sex and P = 0.642 for age) (29). However, in the study by Hu et al., the initial VOR levels were measured on the 7th day of the treatment, while our data shows that the bioavailability of VOR in plasma on the 3rd day was less than the 5th and 7th days (28). These lower levels could be the effect of drug-drug interactions on the metabolism of VOR and chemotherapy medications in the patients. These results prove that the maintenance dose can be prescribed higher in the patients in the first days of drug administration. The concentration of VOR is not constant in the first days of administration, therefore, the 7th day of treatment was suggested as the optimum day for evaluation of VOR concentration.
According to the guidelines of the Infectious Diseases Society of America (IDSA), the VOR dosing for adults is lower than for pediatric patients (24). This dosing regimen indicates that due to the VOR metabolism in pediatrics being higher than in adults, higher VOR doses are administered to pediatric patients (27). These findings are consistent with our data, which indicate that “with each additional year of age, VOR concentration increased by 0.037 µg/mL”, confirming lower metabolism in older age groups. In our study, the most frequent underlying diseases were acute lymphoblastic and acute myeloid leukemia. According to the literature, patients with acute lymphoblastic leukemia, hematologic malignancies, and solid organ transplants are most susceptible to IA (28-30). This data confirms that immunocompromised patients are extensively susceptible to IA. The most frequent site of infection in the present study (65.9%) was the lung, which is consistent with the findings of Hu et al. (28) (90.5%) and Garcia-Vidal et al. (29) (89.5%); and Dib et al. (30) (91%).
In the present study, 77.3% of the patients were treated (complete or partial), which was higher than those reported by Walsh et al. (32) (45%) and Herbrecht et al. (33) (52.8%). Such rates are seemingly associated with the public health system, patient immune status, and the type of Aspergillus in different regions. The total median of VOR concentrations in the present study was 1.79 µg/mL (range of 0.40 - 7.02 µg/mL). The initial median concentrations of VOR in the study by Hu and coworkers (28) were reported to be 1.43 µg/mL (range 0.02 - 9.35 µg/mL).
Therapeutic failure and toxicity were considered when the serum VOR concentration was less than 1.0 µg/mL and more than 5.5 µg/mL, respectively. Nevertheless, these phenomena may be present in other therapeutic ranges of serum VOR concentrations. In our study, VOR-related adverse effects were observed in 4.5% of the patients, with headache, skin rash, visual disturbance, and convulsions. In the study by Martin et al. (27) adverse events such as hepatic abnormalities, visual disturbances, skin complaints, and insomnia were present in 48% (15/31) of patients. In a study by Herbrecht et al. (33) among 194 VOR-treated patients, transient visual disturbances occurred in 44.8% of patients, and hallucinations/fever or both were recorded in 6.7% and 3.1% of patients, respectively. Additionally, skin rash was observed in 8.2% of the patients (33). These reports suggest that VOR plasma concentration plays an important role in avoiding toxic reactions and improving patient management.
The genotypes of CYP2C19 in the studied population were CYP2C19*1*1, CYP2C19*1*17, CYP2C19*1*2, and CYP2C19*2*17 with EM and HEM phenotypes. In patients with invasive fungal infections, the average serum VOR concentration tends to be higher in CYP2C19 PMs and HEMs (27, 34). VOR concentrations were significantly higher in HEMs (P = 0.045) and PMs (P = 0.002) and significantly lower in URMs (P = 0.027) in liver transplant recipients (35). These data support the role of liver enzyme activity; for example, PMs and HEMs lead to higher VOR levels due to reduced enzyme activity. CYP2C19 is an important pathway for VOR metabolism, but other pathways, such as CYP3A4 and CYP2C9, are involved in metabolism. Therefore, VOR dose adjustment based on the CYP2C19 genotype is unacceptable and not recommended.
Evaluation of CRP and ESR concentrations can provide significant information regarding the diagnosis and follow-up of infection and inflammation in patients. VOR is widely metabolized by cytochrome P450 isoenzymes (36). During infections or inflammation, cytochrome P450 isoenzymes can be downregulated, resulting in reduced VOR metabolism, increased levels of VOR, and subsequent toxic responses to VOR (31). Therefore, the pharmacokinetics of VOR are influenced by inflammation (37). In the present study, CRP significantly influenced the concentrations of VOR on the 3rd day of administration (P < 0.001), which indicates that by initiating VOR therapy the higher inflammation confirmed by CRP values affects the VOR metabolism and consequently its bioavailability. However, during the maintenance dose of VOR and improvement of the inflammation and infection, the responsible metabolizing enzyme becomes regulated and there is no relation between CRP and VOR concentration in the following days. The results are similar to Van Wanrooy et al. (37), who reported a significant relationship between the enhancement of CRP and VOR concentration in patients.
The evaluation of liver enzymes, such as ALKP, ALT, AST, DBil, TBil, and Alb, by statistical tests demonstrated that only ALT had a significant effect on the trough levels of VOR. A study by Hu et al. (28) on hematologic patients reported no significant relation between liver function variables (ALT, AST, DBil, TBil, and Alb) and VOR concentrations (P = 0.204, 0.527, 0.050, 0.068, and 0.884, respectively), while ALKP was not considered. In contrast, in a study conducted on allogeneic HSCT recipients, VOR concentrations correlated with ALKP (P = 0.03) and AST (P = 0.0009), but not with ALT and bilirubin (12). There is limited data in the literature that discusses such relationships in detail. Further investigations on the relationship between liver enzymes and VOR concentrations are required. Co-administration of omeprazole, pantoprazole, and prednisolone was reported to have a significant effect on VOR serum concentrations (P < 0.001) (35). In the present study, few patients were treated with the co-administration of diazepam, midazolam, cyclosporine, phenytoin, omeprazole, pantoprazole, or prednisolone; therefore, no statistical alteration in VOR concentration was observed. However, as reported, influential factors, such as administration routes and co-administration with proton pump inhibitors (PPIs), could explain 55.3% of the variability in VOR exposure (28).
5.1. Conclusions
The VOR concentration is diverse in hematologic pediatric patients, despite the same drug dose. According to our results, age, liver function, and CRP level (on the 3rd day of voriconazole therapy) influenced the VOR concentration. In addition, most pediatric treatment cases (partial and complete) were within the normal range of serum VOR concentrations. Therefore, VOR therapeutic drug monitoring is an important strategy for managing pediatric patients, decreasing adverse events, and improving patient outcomes.