1. Background
Urinary tract infections (UTIs) are highly prevalent, impacting over 404.6 million people globally in 2019 and contributing to an estimated 236,786 deaths annually (1, 2). High recurrence rates and increasing antimicrobial resistance among the causative pathogens amplify the long-term economic threat (3-5). The etiological agents most commonly responsible for UTIs include Escherichia coli, Klebsiella species, Staphylococcus saprophyticus, Enterococcus faecalis, and Proteus mirabilis (6). Streptococcus agalactiae (group B Streptococcus or GBS), a gram-positive (GP) bacterium, commonly colonizes the human gastrointestinal and female genitourinary tracts asymptomatically (7). Associated with infections among neonates, pregnant women, the elderly, and immunocompromised individuals, it causes nearly 1 - 2% of all UTIs (8).
Group B Streptococcus can colonize the bladder and urothelium in vivo, indicating invasion into host cells and subsequent destruction of bladder cells (9). Penicillin and aminopenicillins remain the primary treatments of choice for GBS infection. Macrolides and lincosamides are alternatives for patients allergic to β-lactam antibiotics (10, 11). β-lactam antibiotics remain effective against GBS. Despite this, it has been reported that both invasive and non-invasive isolates are exhibiting a rise in their minimum inhibitory concentrations (MICs) against penicillin or ampicillin (12-14). Global antibiotic resistance is increasing, notably against macrolides, lincosamides, fluoroquinolones, and tetracycline (15-17). Vancomycin serves as the last-resort antibiotic for penicillin-allergic patients or when second-choice drugs fail (17). Regular monitoring of GBS isolate susceptibility patterns is crucial.
Group B Streptococcus-caused UTIs encompass various conditions, including asymptomatic bacteriuria, cystitis, pyelonephritis, urethritis, and urosepsis (18). Approximately 7% of pregnancies may face complications due to GBS urinary tract infection, with up to 10% of pyelonephritis cases in pregnant women associated with this infection (19, 20). A recent meta-analysis reported an 18% prevalence of rectovaginal GBS colonization during pregnancy, varying globally, with a high prevalence in the Caribbean (35%) and lower rates in Southern (13%) and Eastern (11%) Asian countries (21). Pregnant women with GBS bacteriuria are more prone to intrapartum complications, including fever, chorioamnionitis, preterm delivery, and premature rupture of membranes (PROM) (22). The risk of vertical transmission to newborns contributes to increased perinatal infections such as chorioamnionitis, stillbirth, preterm birth, neonatal sepsis, and meningitis, leading to increased neonatal mortality (23, 24). The Centers for Disease Control and Prevention (CDC), American Academy of Pediatrics, and American College of Obstetricians and Gynecologists recommend routine GBS screening in the third trimester, followed by appropriate antibiotic prophylaxis, reducing infant mortality rates (25).
While the incidence of GBS neonatal disease has decreased in the past decade, the epidemiology of invasive GBS infections has increased in non-pregnant adults, especially with increasing age (26). Additionally, among non-pregnant individuals affected by GBS invasive infection, nearly 95% had at least one underlying condition, often obesity or diabetes mellitus (25). Though S. agalactiae causing UTIs among pregnant women has been well documented, its prevalence among males and non-pregnant women is infrequent. Despite its uropathogenic nature, there is limited understanding of its clinical and microbiological features, including contributing risk factors (27). Additionally, there is sparse data on the overall prevalence of GBS UTI in the general adult population. Hence, it is vital to determine GBS UTI prevalence, clinical presentation, and associated risk factors among pregnant and non-pregnant adults at our tertiary care center in this region.
2. Objectives
We investigated the prevalence, clinical presentation, antibiotic susceptibility, and risk factors associated with UTIs caused by S. agalactiae in a tertiary care center in India.
3. Methods
This prospective cross-sectional study, spanning 16 months from June 2018 to September 2019, was conducted in a tertiary care hospital in South India. The study included adult patients aged 18 years and above suspected of having UTIs, with confirmed mid-stream urine sample cultures revealing significant growth (≥ 10,000 colony-forming units/mL) of S. agalactiae, a GBS. Among symptomatic patients, urine culture growth of ≥ 1,000 colony-forming units/mL was deemed significant. Patients with negative or nonsignificant urine cultures, as well as those with polymicrobial infections, were excluded from the analysis.
Identification of isolates involved morphological assessment on blood agar (HiMedia Laboratories Ltd., Tarnaka, India), cystine lactose electrolyte deficient agar plate (CLED) (HiMedia Laboratories Ltd., Tarnaka, India), gram staining, biochemical tests, and grouping using a latex agglutination kit (Thermo Fisher Scientific, Waltham, USA). Quantitative urine culture plating was performed on blood agar and selective/differential CLED agar, with incubation at 37°C overnight in ambient air for CLED plates and in a CO2 jar for blood agar plates. The colonies on blood agar exhibited β hemolysis and appeared as small, yellow, opaque colonies on CLED. Microscopic examination of the Gram stain revealed the presence of GP cocci in chains. Biochemical tests indicated the absence of catalase production, and esculin was not hydrolyzed in the presence of bile.
The isolates were tested for resistance to bacitracin (HiMedia Laboratories Ltd., Tarnaka, India). To perform the susceptibility test to bacitracin (0.04 units), a heavy inoculum was made on 5% sheep blood agar using a fresh culture of the suspected organism and incubated at 37°C in CO2 on a 5% sheep blood agar plate. The plates were inspected the next day to determine if there were any zones of inhibition around the disks. The test was interpreted as negative or resistant when the growth reached the edge of the disk (28).
The Christie-Atkins Munch-Petersen (CAMP) test was also carried out on a 5% sheep blood agar plate as per the test protocol of the American Society of Microbiology (2). A narrow streak of S. aureus, known for its abundant β-toxin production, was inoculated onto a sheep blood agar plate using a loop or needle edge. Subsequently, at a right angle to it, a streak of the suspected test organism (GBS) was applied, ensuring it was within 2 mm but not touching the S.aureus streak. After incubating the plates at 35°C for 24 hours in ambient air, a positive result was indicated by the presence of an enhanced "arrowhead-shaped" zone of hemolysis between the two cultures. This distinct hemolytic zone resulted from the synergistic interaction between the two cultures. All isolates tested positive for the CAMP test (29).
Further confirmation using the Streptex™ Latex Agglutination Test with group-specific antibodies (Thermo Fisher Scientific, Waltham, USA) verified the isolates as GBS, following the kit insert protocol. Gram-positive identification cards of the VITEK-2 automated identification system (BioMérieux, France) were employed to confirm isolates with a 98% – 99% probability. Antibiotic susceptibility was determined using the Kirby-Bauer disk-diffusion method on mueller-hinton agar (MHA) supplemented with 5% sheep blood. The tested antimicrobial agents included penicillin (10 U), erythromycin (15 µg), clindamycin (2 µg), ofloxacin (5 µg), cefotaxime (30 µg), linezolid (30 µg), and vancomycin (30 µg) (HiMedia Laboratories Ltd., Tarnaka, India). Susceptibility testing and interpretation followed Clinical Laboratory Standards Institute (CLSI) guidelines (30). Detection of inducible clindamycin resistance (ICR) was carried out using the D test method. Briefly, MHA supplemented with 5% sheep blood was inoculated with a colony suspension of 0.5 McFarland turbidity. The disks were placed 12 mm apart edge-to-edge and contained 15 µg of erythromycin and 2 µg of clindamycin (HiMedia Laboratories Ltd., Tarnaka, India). The plates were incubated for 20 hours at 37°C with 5% CO2. Iinducible clindamycin resistance was defined as the flattening of the zone of inhibition adjacent to the erythromycin disk (referred to as a D-zone).
Data retrieved from electronic hospital records included clinical details such as fever duration, dysuria, flank pain, lumbar tenderness, risk factors, complications, morbidity, and mortality. A provisional diagnosis of urinary tract infection was based on significant S. agalactiae growth (≥ 10,000 CFU) along with at least one symptom, such as fever (> 38°C), dysuria, flank pain, increased frequency or urgency, and lumbar tenderness. Pure significant growth of GBS in both symptomatic and asymptomatic cases was considered positive. Information about the departments sending patient samples was also recorded. A waiver of consent was obtained from the Institute Ethical Committee since the data was collected solely from electronic records without direct patient contact.
3.1. Statistical Analysis
Microsoft Excel 2019 was used for data analysis, and results were expressed descriptively. The chi-square test was employed to determine the significance of the observed distribution of risk factors compared to an expected equal distribution among patients with S. agalactiae UTIs. Additionally, a z-score calculation was performed to compare the prevalence rate of S. agalactiae UTIs observed in the studied population with the reported prevalence rate in Southwest India.
4. Results
Over the course of the study, a total of 6305 patient records were accessed from the hospital electronic records according to the study inclusion criteria. The patients' records were scrutinized, and the information was recorded in a Microsoft Excel sheet. Details such as demographic data, history of presenting complaints related to urinary infections, and coexisting illnesses were recorded. Information regarding the specialty consulted and pathological and biochemical investigation details of both blood and urine were also documented. To compile the information, the records of patients who had undergone urine culture and antibiotic susceptibility testing were carefully scrutinized and tabulated. Among these, 134 adults were found to be culture-positive for urinary tract infection due to significant growth of S. agalactiae.
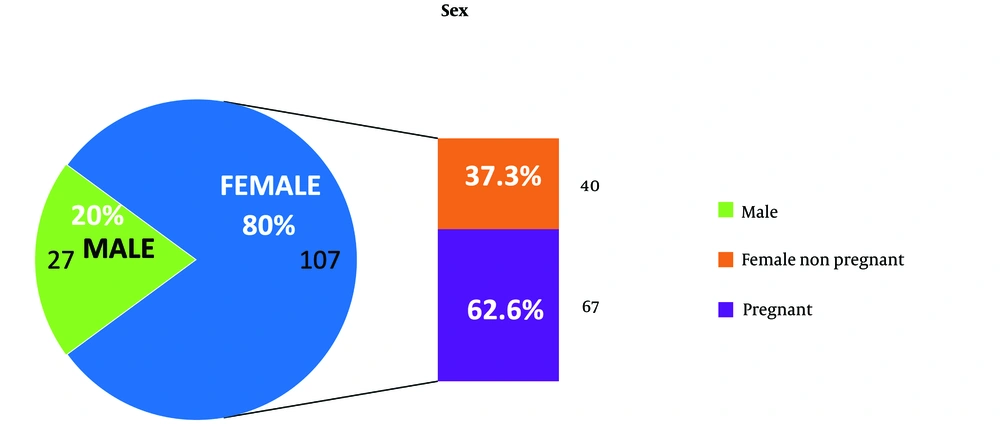
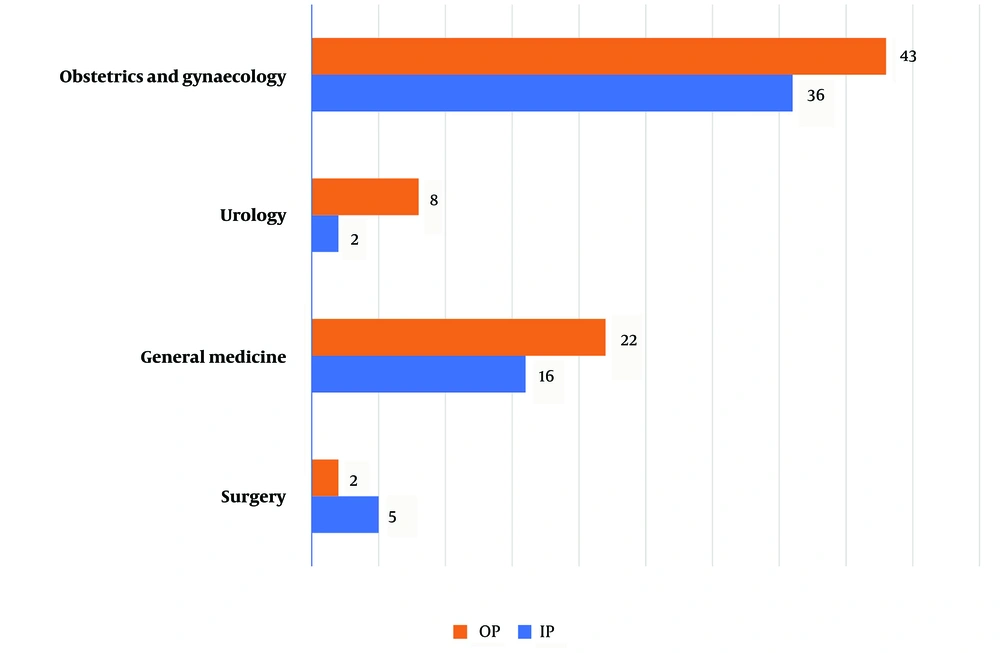
The prevalence rate of S. agalactiae was found to be 2.1% (134/6305) with a 95% confidence interval (CI) of 0.68% - 4.8%. Out of the 134 isolates, 75 were referred from outpatient clinics and 59 were from patients admitted to the hospital under various specialties. Among the 134 patients, 80% (107) were female. The remaining 20% (27) were male patients who had confirmed S. agalactiae UTIs. Among the 107 female patients, 79 were referred from the Department of Obstetrics and Gynaecology, with antenatal cases accounting for the majority (N = 67) identified during their routine antenatal visits to the hospital (Figure 1). The second most common referral with confirmed S. agalactiae urinary tract infection was from the Department of General Medicine, with 38 (28%) patients. The remaining 12.7% (17/134) of patients with S.agalactiae UTI were referred from surgical specialties such as the Departments of General Surgery and Urology (Figure 2).
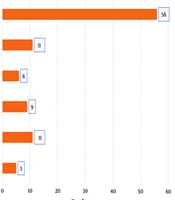
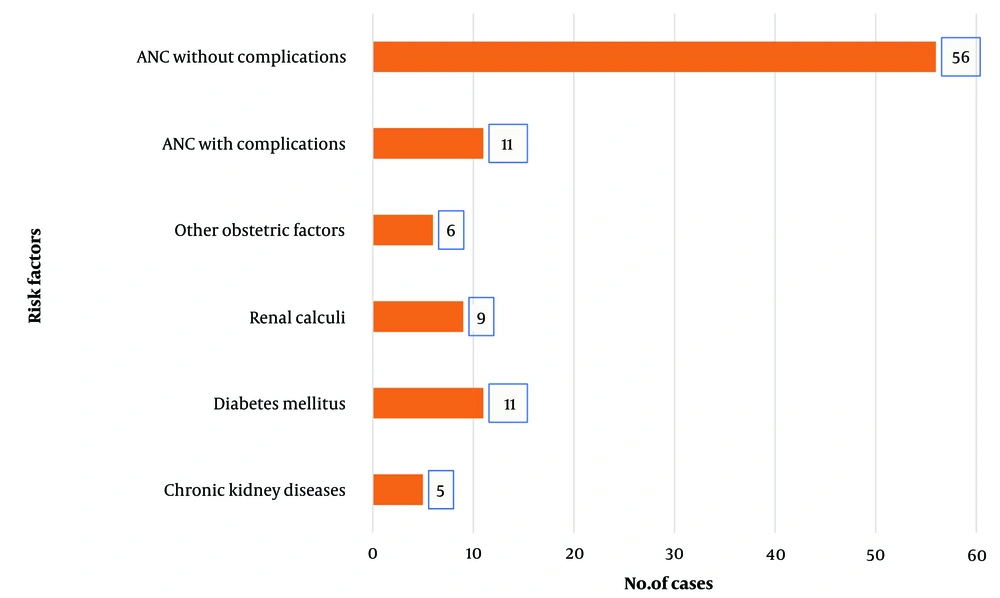
Concerning the risk factors associated with S. agalactiae urinary tract infection, it was identified that 67 patients, which is almost 50% of S. agalactiae positive urinary tract infection isolates, were antenatal cases, highlighting high-risk pregnancy. The remaining risk factors were identified as other obstetric factors (apart from pregnancy), diabetes mellitus, chronic kidney disease (CKD), and renal calculi, more commonly identified in the male population (Figure 3). The chi-square test was employed to assess the distribution of various risk factors associated with S. agalactiae UTIs (Table 1). By comparing the observed frequencies with the expected frequencies, assuming equal distribution, the analysis revealed a significant deviation (P < 0.0001). The low P-value suggests that certain factors, such as antenatal care without complications, exhibit a markedly higher prevalence than expected by chance alone. This underscores the importance of targeted interventions, particularly in antenatal care, to address the heightened risk associated with specific factors and effectively manage and prevent UTIs caused by S. agalactiae.
| Risk Factors | Chi-square | P-Value |
|---|---|---|
| Chronic kidney disease | 7.86 | < 0.0001 |
| Diabetes mellitus | 1.74 | 0.1841 |
| Renal calculi | 3.29 | 0.5097 |
| Other obstetric factors | 6.53 | 0.2608 |
| ANC with complications | 1.74 | 0.1841 |
| ANC without complications | 93.10 | < 0.0001 |
Association of Risk Factors in Streptococcus agalactiae Urinary Tract Infections
This table provides a clear overview of the chi-square statistics and P-values for each risk factor. It indicates the significance level for each risk factor contributing to the observed distribution of S. agalactiae UTIs. Lower P-values suggest greater significance.
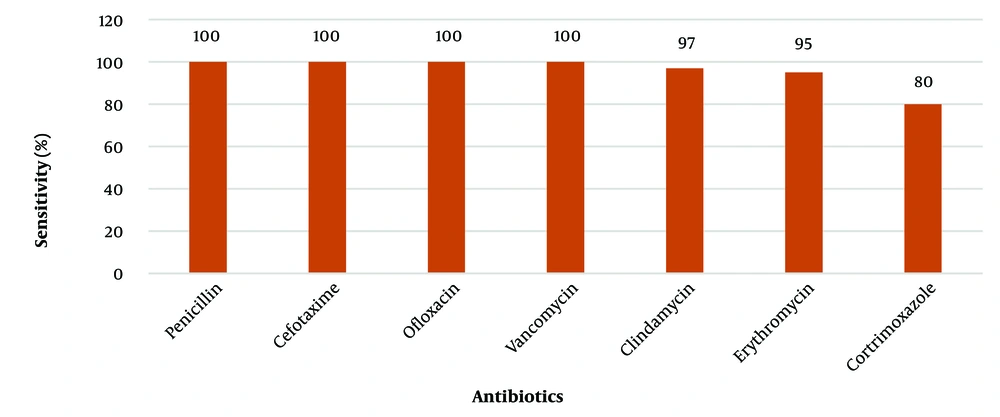
All 134 GBS isolates were found to be sensitive to penicillin, cefotaxime, ofloxacin, linezolid, and vancomycin. The sensitivity rates for cotrimoxazole, erythromycin, and clindamycin were 106/134 (80) %, 127/134 (95) %, and 129/134 (97) %, respectively (Figure 4). Iinducible clindamycin resistance was not observed among these isolates. Among the 134 cases, antenatal cases without complications (41.7%, N = 56) and non-pregnant women and adults (33.5%, N = 45) were treated with a course of either 3rd or 4th generation cephalosporins. The remaining patients (24.6%, N = 33) with predisposing comorbid conditions such as CKD, renal calculi, diabetes mellitus, and obstetric complications (placenta previa, threatened abortion, PROM, and gestational diabetes) were treated with intravenous vancomycin.
5. Discussion
Streptococcus agalactiae infection of the urinary tract is an uncommon cause of UTIs. Over the past decade, the prevalence of genitourinary infections caused by GBS has been on the rise (31). This study was conducted to identify the prevalence of S. agalactiae urinary infections and their associated risk factors. Based on the analysis of 6305 urine specimens cultured during the study period, it was determined that the prevalence rate of UTIs caused by GBS during this study is 2.1% (134) with a 95% CI of 0.68% - 4.8%. According to this study, the prevalence rate for the disease is higher than the prevalence rate for Southwest India, which is only 0.27% (32). The prevalence rate and z-score analysis, with a z-score of 89.27 far exceeding the critical value of 1.96 at a 0.05 significance level, revealed a significant difference between the studied population and the regional report. This 2.1% population included a larger proportion of pregnant women (62.6%, N = 67) as shown in Figure 1. The higher prevalence rate might be due to effective screening as a part of routine pregnancy investigations in this part of the country. This aligns with the CDC recommendation for universal screening with GBS rectovaginal culture between 35 to 37 weeks in each pregnancy (33).
GBS-associated UTIs in males are not well documented compared to females (34). In this study, 27 (20%) of cultures from male patients reported UTIs due to GBS. A study conducted by Sewaify et al. showed that GBS was the responsible pathogen in around only 1% of the urinary tract infection cases in male patients (35). Studies show that the most frequent risk factors associated with GBS bacteriuria were CKD (30.8%) and diabetes mellitus (15.4%) (36). The elevated prevalence of males observed in this study may be attributed to factors such as the occurrence of CKD, renal calculi, or diabetes within this particular patient subgroup, as illustrated in Figure 3. Furthermore, the specialized nephrology unit in this tertiary care hospital could also play a role in shaping the observed gender distribution. A greater likelihood of diabetic patients having UTIs caused by GBS is attributed to glycosuria, neutrophil dysfunction, and increased adherence of bacteria to the uroepithelium in diabetic patients (32). In this present study, 11 (0.08%) patients were diabetics with GBS-caused UTIs, which is far less compared to the prevalence rate of 5.6% reported by Paudel et al. (37). This may be a limitation of our study design, where effective selection criteria for screening diabetes were not implemented.
The higher prevalence of GBS during pregnancy identified in this study is of great concern as it requires appropriate measures to prevent vertical transmission to neonates. During pregnancy and puerperium, physiological changes contribute to urinary stasis and a vesicoureteral reflex, increasing susceptibility to UTIs (38). A total of 79 patients (59%) out of 134 cases were reported to have positive urine cultures for GBS from the Department of Obstetrics and Gynaecology. Studies also identified the prevalence of colonization among the Indian population in pregnant women, varying from 2% to 62%. Transmission of GBS infection from pregnant women to their newborns was reported in two studies, varying from 6.7% to 11.1% (39). This high prevalence rate among pregnant women might lead to early-onset and late-onset GBS infections like pneumonia, meningitis, and sepsis in newborns. The importance of early detection of GBS during pregnancy was emphasized by the National Guidelines for Infection Prevention and Control in Healthcare Facilities, India, adapted from WHO. It recommends a risk-based approach for prophylaxis of GBS infection and intrapartum antibiotic prophylaxis (IAP) in women with GBS colonization to prevent early neonatal GBS infection (39). Out of the 134 cases, individuals without complications during antenatal care (41.7%, N = 56) and non-pregnant women and adults (33.5%, N = 45) received a treatment course comprising either 3rd or 4th generation cephalosporins. After completing the antibiotic course, a repeat urine culture was conducted two weeks later to confirm that the cultures were negative.
Other risk factors associated with GBS UTIs identified in this study included recurrent abortion, placental hemorrhage, gestational diabetes, PROM, abnormal uterine bleeding, fibroid uterus, and menorrhagia, as shown in Figure 3. Globally, 8 - 11% of all preterm births, neonatal sepsis, and stillbirths have been attributed to GBS UTIs during pregnancy. During pregnancy, 15 - 30% of women are colonized, and vaginal colonization poses a significant risk for preterm birth, neonatal sepsis, and stillbirth (40). Namavar et al. reported higher incidences of prolonged rupture of membranes and preterm PROM among mothers colonized with GBS during pregnancy (41). Group B Streptococcus isolates from clean-catch urine samples during any pregnancy trimester, regardless of colony count, can serve as a surrogate for heavy vaginal-rectal colonization. Regular rectovaginal screenings for GBS during the antepartum period are recommended, followed by IAP for positive cases. This helps prevent complications related to GBS-caused UTIs (42).
In our study, the antibiotic susceptibility testing of S. agalactiae samples revealed that all 134 isolates were uniformly sensitive to β-lactam antibiotics like penicillin and cefotaxime, and glycopeptide antibiotics like vancomycin. This finding was consistent with several other reports worldwide (43-45). While 20% of the isolates were resistant to cotrimoxazole, 3% to clindamycin, and 5% to the macrolide antibiotic erythromycin. A study done in Kuwait showed that 8% of the S. agalactiae isolates were resistant to cotrimoxazole, and 15% were resistant to clindamycin and erythromycin, which was comparable to our study (35). A higher range of resistance to clindamycin and erythromycin was reported by a few other studies, ranging from 16.4% to 70% (46-48). Resistance to macrolides in S. agalactiae is primarily due to erm (B) or macrolide efflux genes mef (A).
The expression of the erm (B) gene is associated with a wide range of resistance toward macrolides, lincosamides, and streptogramins B (MLSB phenotype), while the mef (A) expression is associated with low levels of resistance to macrolides only (M phenotype) (44). This information helps choose the antibiotic treatment in cases of induced resistance to clindamycin. Based on the results of our study, none of the isolates exhibited induced resistance to clindamycin. Few studies have reported ICR among S. agalactiae with rates ranging from 3% to 17.4% (49, 50). Antimicrobial resistance is prevalent in S. agalactiae globally, but the percentage of antimicrobial resistance varies depending on the region and period in which the study was conducted. Macrolides and lincosamides are commonly used as alternatives to penicillin in allergic patients with GBS infections (51). Consequently, continuous screening for the resistance of S. agalactiae to these antibiotics is necessary.
5.1. Conclusions
Invasive GBS UTIs present challenges in both pregnant and non-pregnant adult populations. Risk factors include pregnancy, obstetric factors, renal calculi, and chronic medical conditions like CKD and diabetes mellitus. Routine screening of pregnant women during 34 - 37 weeks of pregnancy, along with antibiotic susceptibility testing, aids in the early identification and prevention of S. agalactiae UTIs and associated newborn complications. Men are also susceptible to GBS UTIs, with early detection crucial for preventing complications related to chronic conditions. Awareness and routine culture and susceptibility testing for GBS in UTI cases are essential to address emerging antibiotic-resistant strains and ensure effective treatment.