1. Background
Bacterial infections resistant to available antibiotics have become a major health problem worldwide. These infections are severe, requiring expensive diagnostic methods and prolonged treatments (1, 2). Antibiotic resistance is a global and increasing threat to human health, with excessive use of antibiotics being one of the primary causes (2-5). Antibiotic resistance depends on both the quantity and inappropriate use of antibiotics. It remains unclear why antibiotic resistance is spreading rapidly in developing countries (6). The role of factors such as administering antibiotics to promote growth in livestock, the release of antibiotics into urban wastewater by pharmaceutical manufacturers, or poor hygiene practices is not well understood (7).
In European countries, particularly in Scandinavian nations where antibiotic use is lower, antibiotic resistance is also lower (4, 8). Antibiotic resistance is associated with healthcare-associated infections (9). Strategies to control antibiotic use, including limiting antibiotic prescriptions and providing proper education, have significantly impacted the reduction of antibiotic prescriptions (10). Without mandatory guidelines, physicians are often hesitant to shorten the prescription period or limit their empirical antibiotic choices (5). Studies in Europe and the United States have shown that limiting antibiotic use was implemented correctly in only 11 - 55% of patients (5, 10, 11). Several potential options exist to moderate antibiotic prescriptions and reduce antibiotic resistance (2, 5). In recent years, measures have been introduced to improve antibiotic administration in hospitals (12). Providing evidence for antibiotic prescriptions, rotating antibiotics, and combining antibiotic therapies have been suggested as strategies to reduce antibiotic resistance, although the use of these methods has limitations (3).
The global point prevalence survey of antimicrobial consumption and resistance (GLOBAL-PPS 2020) was conducted under the management of Antwerp University, Belgium, with the aim of improving the prescription of antibiotics in hospitalized patients. This project focuses on collecting data on antibacterials, systemic antivirals, antibiotics used in the treatment of tuberculosis, and antibiotics used for intestinal infections in European hospitals and volunteer countries (13). Another important goal of this standard monitoring is to gather data from all European Union countries and other participating nations (73 countries) to track the use of antimicrobial agents in patients across all age groups. Additionally, the World Health Organization (WHO) provided support and solutions to help control and improve the process of antibiotic prescription and manage antibiotic resistance (5). This program identifies the type, dosage, and indications for prescribing antibiotics in hospitalized patients.
2. Objectives
Given that excessive antibiotic prescription is a primary factor in the emergence and spread of resistant bacteria, we have chosen to conduct a longitudinal study in collaboration with the University of Antwerp in Belgium and the WHO. Our objective is to investigate the prevalence of both general and inappropriate antibiotic prescriptions, as well as to evaluate antibiotic resistance in Ahvaz, a city located in the southwest of Iran.
3. Methods
This study was a descriptive longitudinal study conducted on all patients hospitalized in Imam Khomeini, Golestan, and Abuzar (pediatric center) Hospitals of Ahvaz University of Medical Sciences between 2020 and 2021, with a total of 2,035 participants. The study evaluated forty-three hospital wards across the three hospitals using a census method. Inclusion criteria required participants to have received systemic antibiotics and been hospitalized before 8 AM on the day of the study. Data for each hospital were collected over four consecutive weeks, including cases where antimicrobials were prescribed for at least 48 hours, even if discontinued on the day of data collection. Any antimicrobial use that began after the afternoon of the data collection day was excluded from the study.
During the study, information from each ward was collected separately on a specific day. The data collection tool used was checklists. Participants' demographic information, such as age, gender, weight, and specific details including the type of antimicrobial medicines, dosage per administration, number of doses per day, method of administration, main diagnosis, and anatomical site of infection or reason for prophylaxis, were collected according to a list of treatment indications (community-acquired infections, hospital-acquired infections, or prophylaxis). Data collection was guided by the project manager at Antwerp University, Belgium, using internet resources. Information for all hospitalized patients was gathered, with data from each hospital entered into the checklist over a two-week period. This information was then recorded in the online software specific to this study (Global Point Prevalence software).
According to the GLOBAL, antibiotic usage is classified as follows (13):
- Hospitals: Golestan, Imam Khomeini, Abuzar
- Country: Iran
- Continent: East and South Asia
- Hospital type: Tertiary hospitals (major hospitals that typically offer a full range of services, including pediatrics, obstetrics, general medicine, gynecology, various branches of surgery, psychiatry, or specialty hospitals dedicated to specific sub-specialties such as pediatric centers, oncology centers, or psychiatric hospitals. Patients are often referred from smaller hospitals to tertiary hospitals for major surgeries, consultations with sub-specialists, or when advanced intensive care facilities are required).
- Region: Europe
Throughout the study, there was no discussion or personal judgment regarding the appropriateness of antibiotic prescriptions. Statistical analysis was performed by comparing the results with those from other countries. Continuous quantitative variables, such as the mean and standard deviation, were calculated, while qualitative variables were reported as numbers and percentages. The data were analyzed using SPSS 22 and Excel 2016 software.
4. Results
This study was conducted with 2035 participants, of whom 50.51% were male. A total of 41.22% of the participants were from Imam Khomeini Hospital (Table 1). The results showed that the overall frequency of antibacterial use in Golestan and Imam Khomeini Hospitals was 338 (43.2%) and 370 (44.1%), respectively. This rate was reported as 216 (57.9%) in the children's general ward and 36 (86.1%) in the pediatric intensive care unit (PICU) at Abuzar Hospital. Additionally, in this hospital, the frequency of children who received at least one dose of antimicrobial medicine per day was 81 (21.6%) (Table 2).
| Variables and Subgroups | No. (%) or Mean ± SD |
|---|---|
| Gender | |
| Male | 1028 (50.51) |
| Female | 1007 (49.49) |
| Hospital | |
| Imam Khomeini | 839 (41.22) |
| Golestan | 728 (38.42) |
| Abuzar | 414 (20.36) |
| Quantitative variable | |
| Age of participants | 46.18 ± 8.83 |
Demographic Characteristics of Participants
| Variables and Subgroups | Hospitals | ||
|---|---|---|---|
| Golestan (N = 782) | Imam Khomeini (N = 839) | Abuzar (N = 414) | |
| Prevalence | |||
| Total | 338 (43.2) | 370 (44.2) | 216 (57.9) |
| Adults | 151 (31.1) | 104 (27.9) | - |
| Surgery ward | 116 (56) | 25 (52) | - |
| ICU | 71 (47.9) | 187 (40.6) | - |
| Children | 19 (10.5) | 60 (71.7) | - |
| Infection | |||
| Community-acquired | 60 (89.6) | 79 (98.8) | 105 (90.5) |
| Healthcare-associated | 7 (10.4) | 1 (1.2) | 11 (9.5) |
| Site | |||
| Skin and soft tissue | 13 (28.3) | 13 (29.5) | 2 (3.6) |
| Sepsis | 7 (20.6) | 9 (20.5) | 10 (17.5) |
| Nervous system | 3 (8.8) | 2 (4.5) | 1 (1.8) |
| Pneumonia | 2 (5.9) | 3 (6.8) | 20 (35.1) |
| Gastrointstinal | 1 (2.9) | 0 (0) | 3 (5.3) |
| Cardiovascular | 3 (6.8) | 3 (6.8) | 0 (0) |
| Tuberculosis | 3 (6.8) | 2 (4.5) | 0 (0) |
| Osteomyelitis | 3 (6.8) | 0 (0) | 1 (1.8) |
| Upper urinary tract | 0 (0) | 0 (0) | 1 (1.8) |
| Lower urinary tract | 0 (0) | 3 (6.8) | 12 (21.1) |
| Cystic fibrosis | 0 (0) | 0 (0) | 1 (1.8) |
| Prescribing of intravenous antibiotics | 148 (20.3) | 139 (16.5) | 125 (30.1) |
| Prescribing multiple antibiotics | 105 (14.4) | 139 (16.5) | 93 (22.4) |
| Type of treatment | |||
| Experimental | 273 (30.3) | 263 (32.2) | 22 (5.3) |
| Targeted | 11 (1.4) | 2 (0.02) | 20 (4.8) |
| Type of prophylaxis | |||
| Gastrointestinal tract infection | Ceftriaxone | Ceftriaxone/metronidazole | Ceftriaxone/metronidazole |
| Urinary tract infection | Meropenem | Meropenem | Ceftriaxone/amikacin |
| Pneumonia | - | Meropenem | |
| Surgical prophylaxis | - | Ceftriaxone/metronidazole | Ceftriaxone/metronidazole |
Prevalence of Antibiotics and Related Variables
The overall frequency of antibacterial use in adults at Golestan and Imam Khomeini Hospitals was 151 (31.1%) and 104 (27.9%), respectively. In the surgical wards of Golestan and Imam Khomeini Hospitals, the frequency was 116 (56%) and 25 (52%), respectively. In the intensive care units (ICU) of these hospitals, the frequency was 71 (47.9%) and 187 (40.6%), respectively. In the pediatric wards of these hospitals, the frequency was 19 (10.5%) and 60 (71.7%), respectively (Table 2).
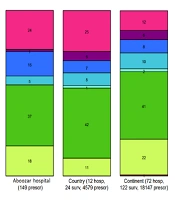
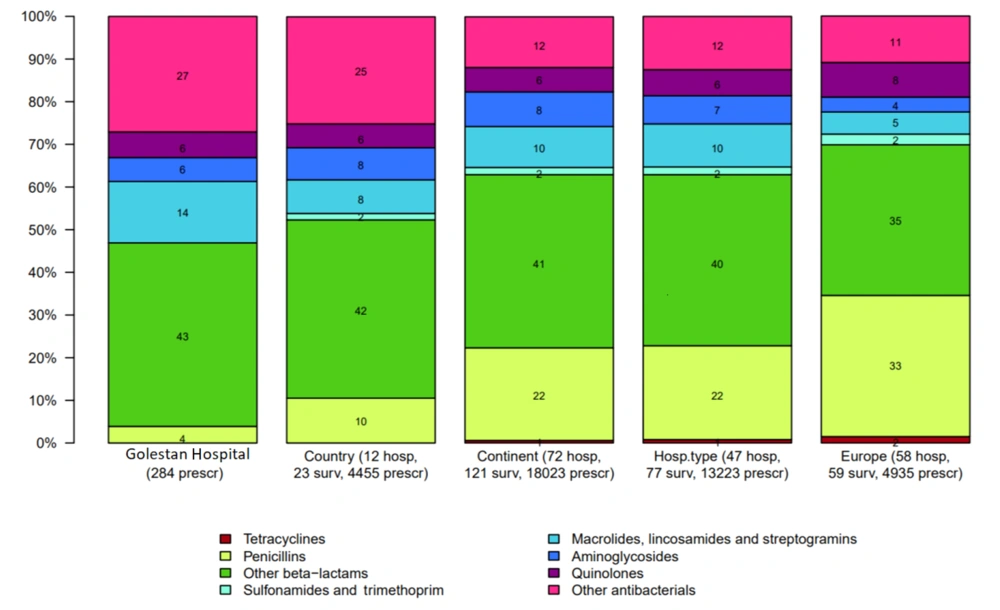
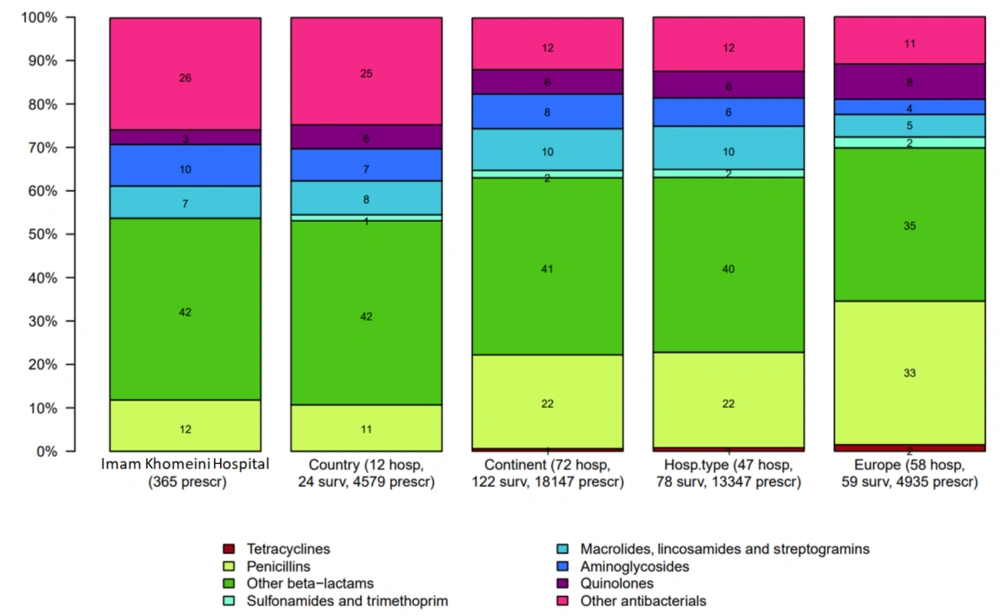
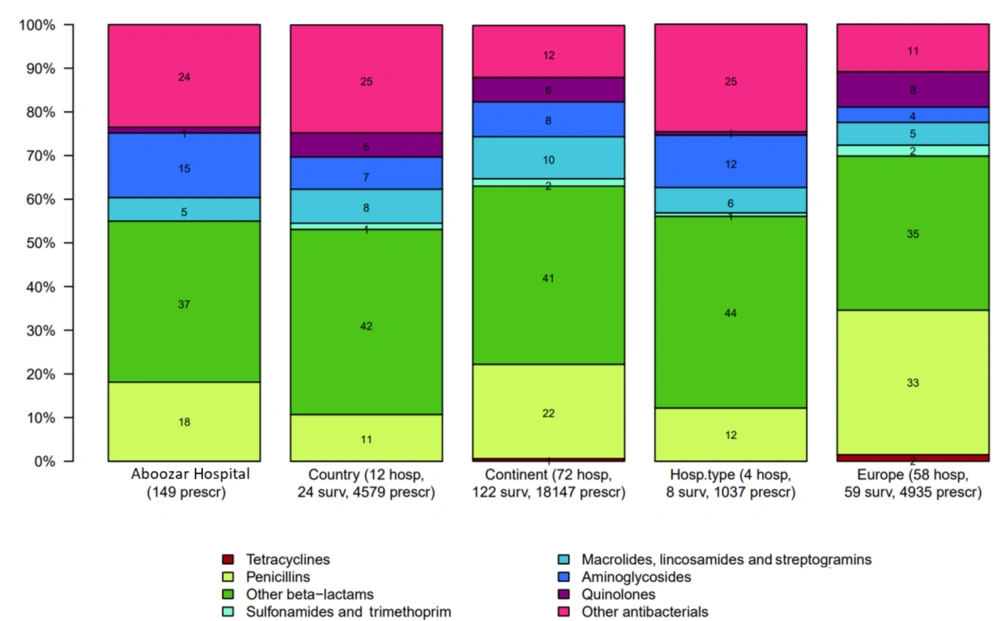
The most commonly used antibiotics in Golestan, Imam Khomeini, and Abuzar Hospitals were beta-lactams (including first to fourth-generation cephalosporins and carbapenems), which accounted for 43%, 42%, and 46%, respectively. Third-generation cephalosporins were the most commonly used antibiotics in all three hospitals, accounting for 28.6%, 20%, and 32.4%, respectively. The least commonly used antibiotics included penicillins (4%), quinolones (3%), and macrolides (5%) (Figures 1 - 3).
The frequency of antibiotic use for community-acquired infections in Golestan, Imam Khomeini, and Abuzar Hospitals was 60 people (89.6%), 79 people (98.8%), and 105 people (90.5%), respectively. For healthcare-associated infections, there were 7 people (10.4%), 1 person (1.2%), and 11 people (9.5%), respectively (Table 2). The most common diagnoses treated with antimicrobial medicine in Golestan and Imam Khomeini Hospitals included skin and soft tissue infections [13 (38.2%) and 13 (29.5%), respectively] and sepsis [7 (20.6%) and 9 (20.5%), respectively]. In Abuzar Hospital, the most common diagnoses were pneumonia [20 (35.1%)] and lower urinary tract infections (UTIs) [12 (21.1%)]. Other results are presented in Table 2.
The most common antibiotic for sepsis in adults and children in Golestan, Imam Khomeini, and Abuzar Hospitals was vancomycin (28.5%, 22.2%, and 40%, respectively). In Golestan Hospital, ceftriaxone was used more frequently for treating gastrointestinal infections, while meropenem was used more often for upper and lower UTIs. In Imam Khomeini Hospital, meropenem was used more for pneumonia treatment, ceftriaxone and metronidazole were used for surgical prophylaxis, ceftriaxone and metronidazole for gastrointestinal infection prevention, and ceftriaxone for urinary tract infection surgical prophylaxis. In Abuzar Hospital, metronidazole and ceftriaxone were used for surgical prophylaxis and gastrointestinal infections, and ceftriaxone and amikacin were used to treat upper and lower UTIs (Table 2).
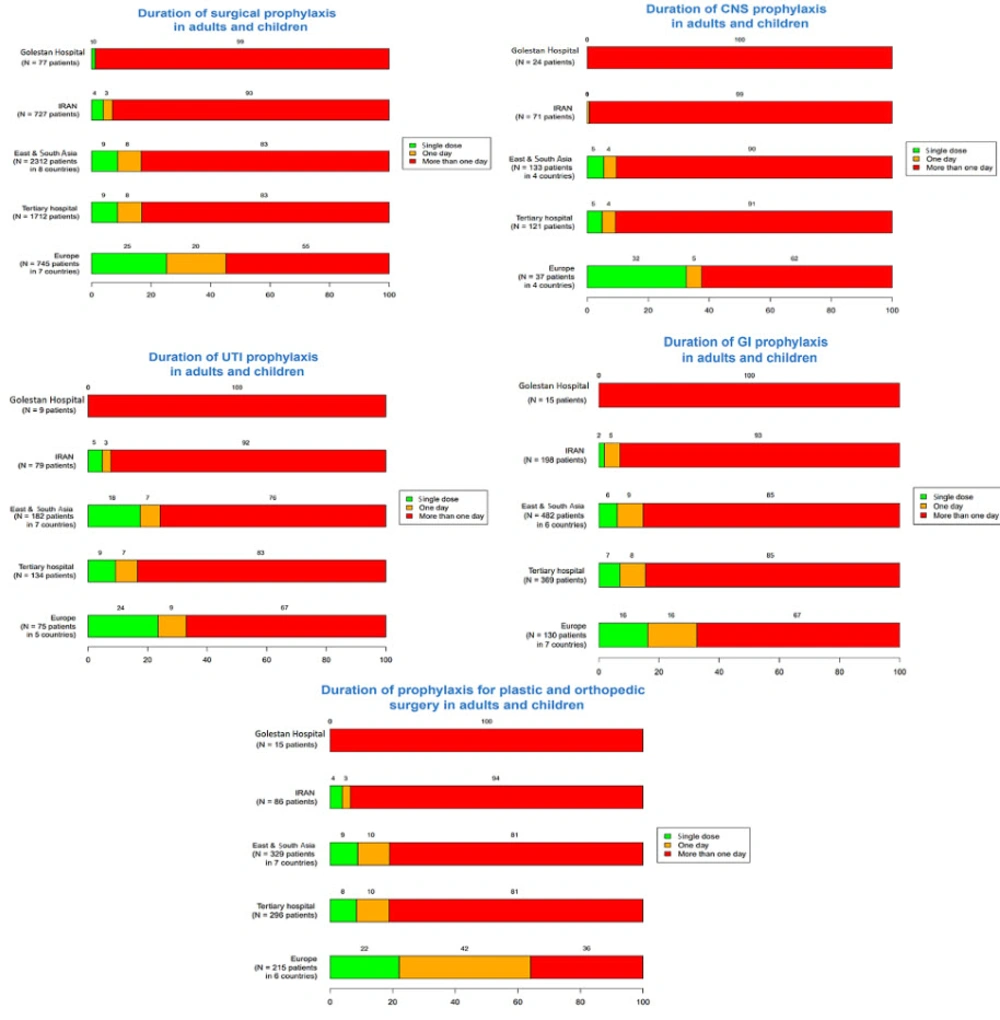
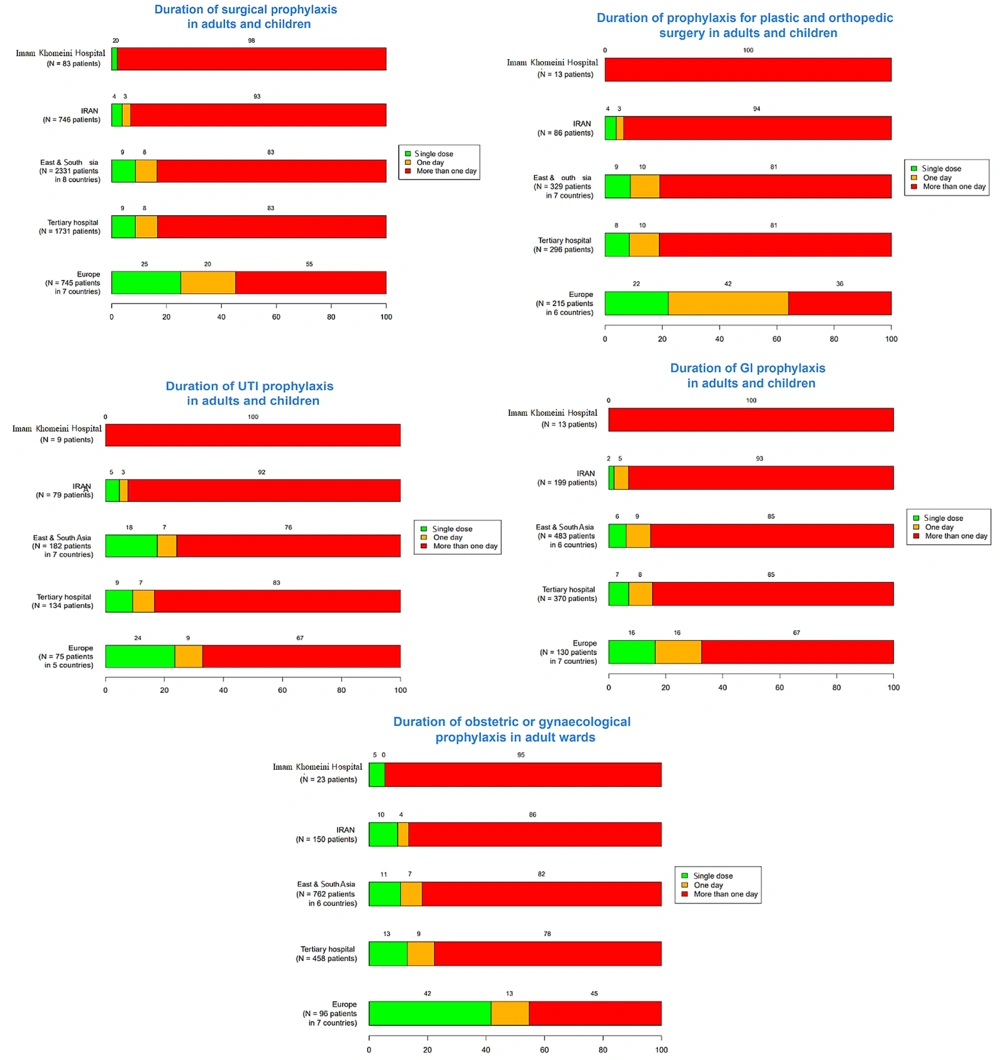
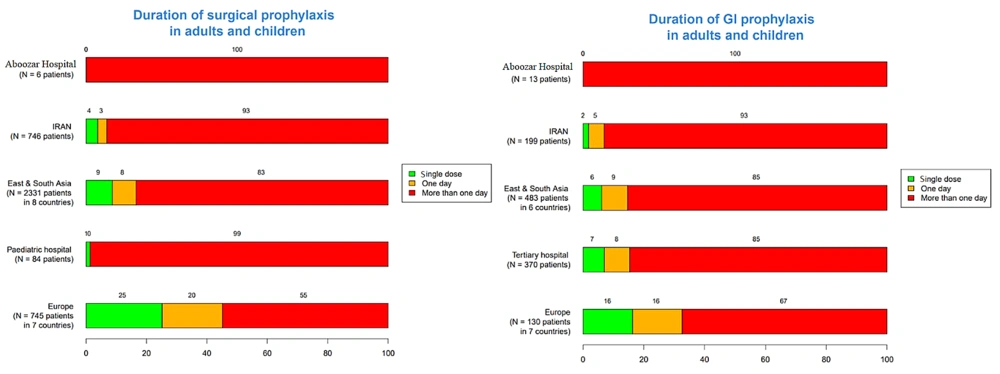
The frequency of a single-dose surgical prophylaxis in adults and children in Golestan and Imam Khomeini Hospitals was 1% and 20%, respectively. This rate was zero for upper UTIs, plastic surgery, orthopedic surgery, gastrointestinal tract infections, and central nervous system infections in adults and children in each hospital. Additionally, in Imam Khomeini Hospital, the frequency of a single dose of prophylaxis in the obstetrics and gynecology ward was reported as 5%, while in Abuzar Hospital, the single-dose frequency of prophylaxis for children's surgery and gastrointestinal infections was reported as 2%. The frequency of intravenous antibiotic prescriptions for all patients in Golestan, Imam Khomeini, and Abuzar Hospitals was 148 (20.3%), 139 (16.5%), and 125 (30.1%) patients, respectively. Also, in the three hospitals, 273 (30.3%), 363 (32.3%), and 22 (5.3%) patients underwent empirical treatment, and 11 (1.4%), 2 (0.2%), and 20 (4.8%) patients underwent targeted antibiotic therapy, respectively (Table 2).
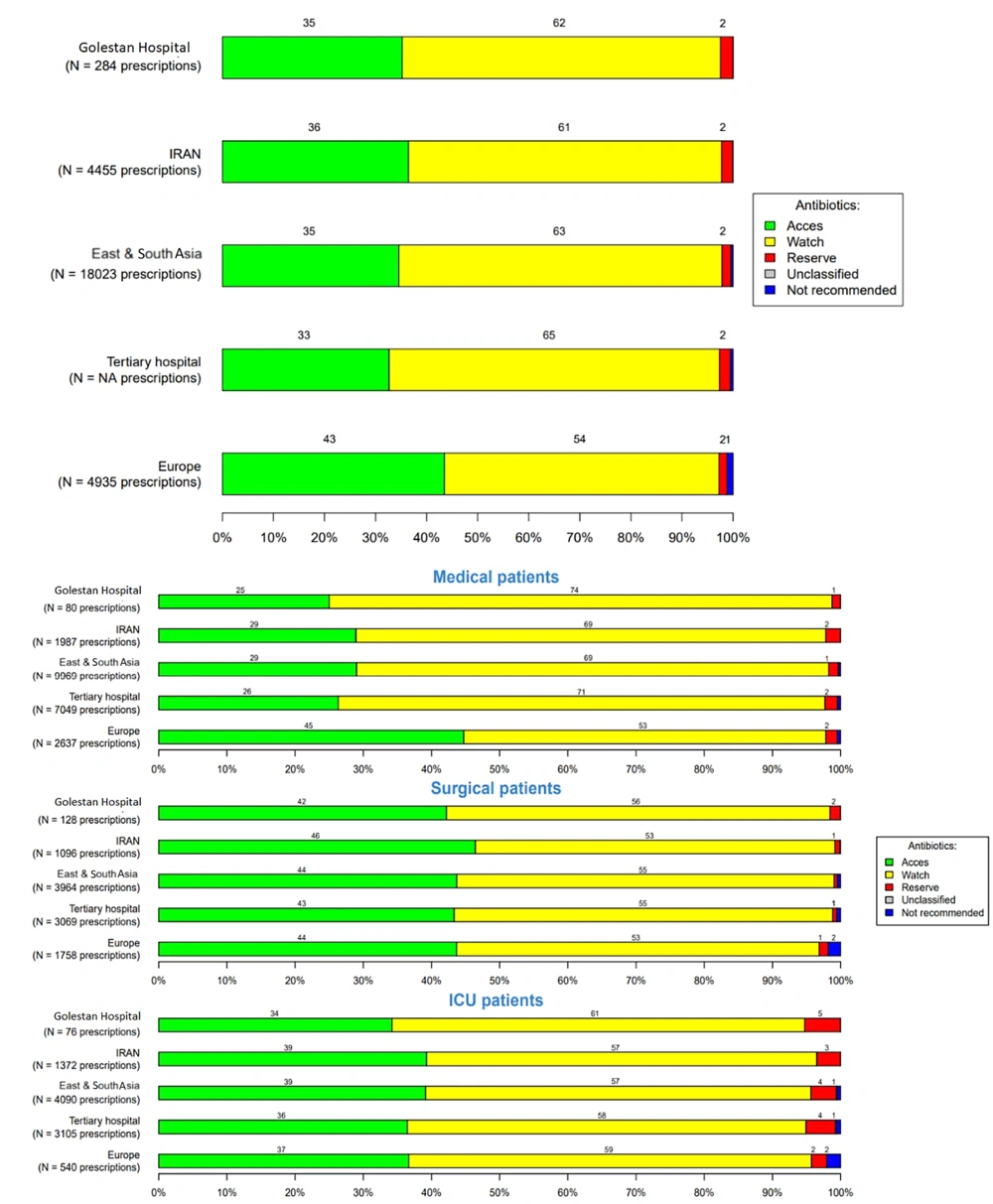
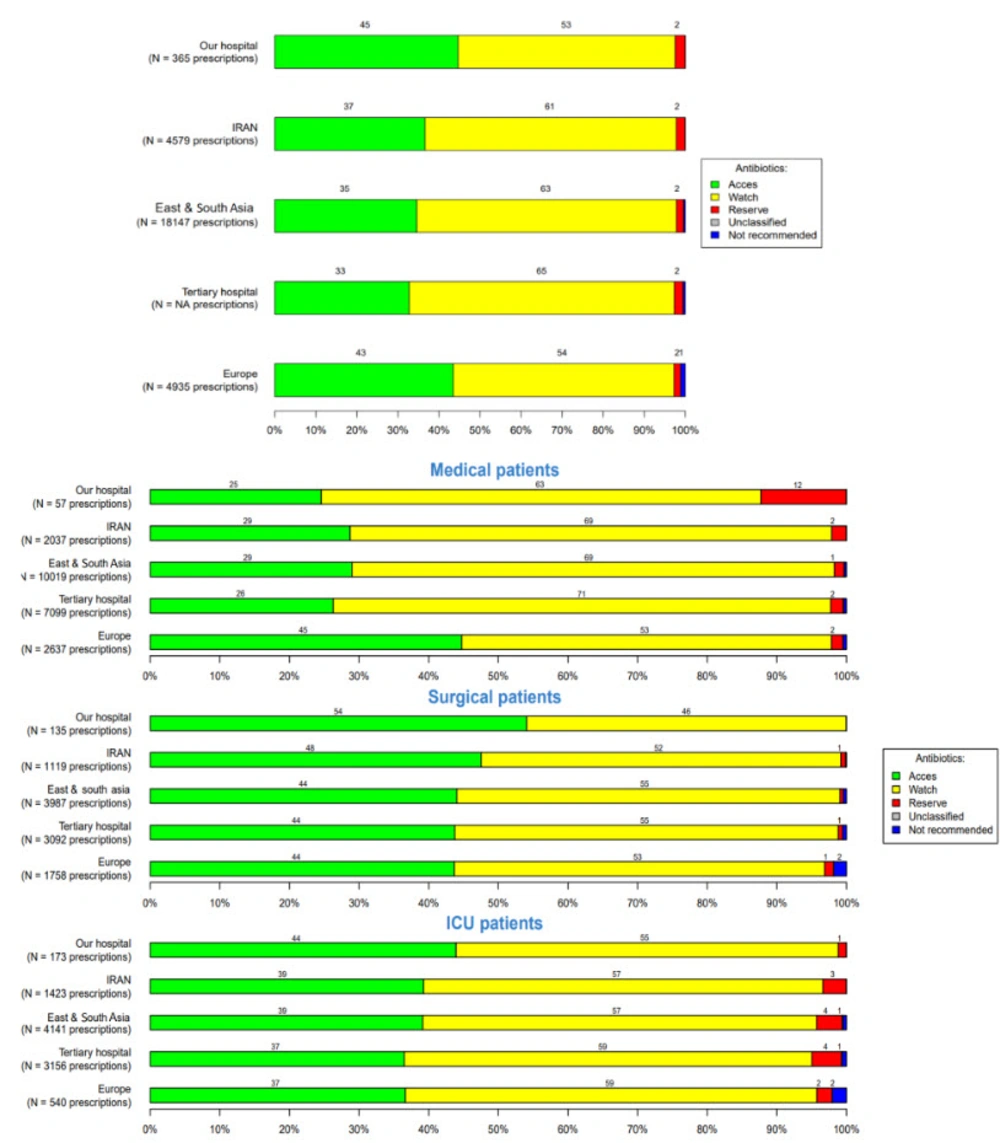
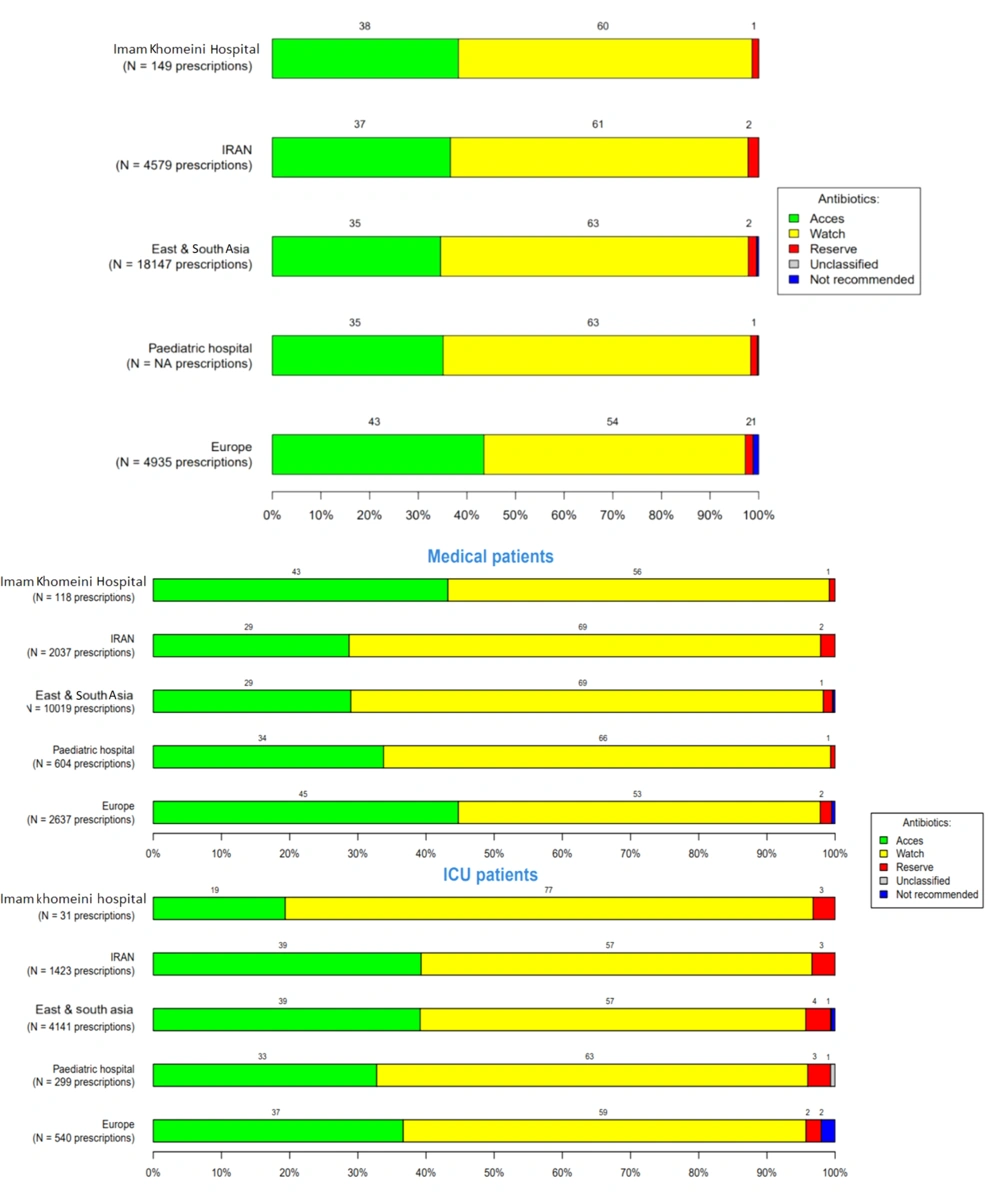
Overall antibiotic prescriptions for systemic use, according to the WHO AWaRe classification (WHO: Access, watch, reserve), in the three hospitals were 62%, 53%, and 57%, respectively, in the Watch group (the watch group contains broad-spectrum antibiotics with a higher potential for developing resistance), 35%, 45%, and 41% in the Access group (the access group contains antibiotics used in the first- and second-line treatment of infections), and 2% in the reserve group (the reserve group contains last-resort antibiotics used for multidrug-resistant infections) (Figures 4 - 6).
The results related to the duration of prophylaxis are presented in Figures 7 - 9. The highest rates of antimicrobial resistance were reported for cefazolin (81.42%), ampicillin (93.77%), and ampicillin (94.02%) in Golestan, Imam Khomeini, and Abuzar Hospitals, respectively. Additionally, the lowest rates of antimicrobial resistance were reported for ciprofloxacin (18.57%) in Golestan Hospital, ciprofloxacin (15.58%) in Imam Khomeini Hospital, and piperacillin/tazobactam (12.04%) in Abuzar Hospital, respectively (Table 3).
| Culture Results | Golestan (N = 70) | Imam Khomeini Hospital (N = 76) | Abuzar Hospital (N = 83) |
|---|---|---|---|
| Ampicillin | 35 (50) | 71 (93.77) | 67 (94.02) |
| Cefazolin | 57 (81.42) | 32 (43.33) | 32 (38.55) |
| Ciprofloxacin | 13 (18.57) | 12 (15.58) | 14 (16.96) |
| Amikacin | 28 (40) | 30 (39.47) | 33 (39.75) |
| Gentamicin | 25 (35.71) | 27 (35.52) | 29 (34.93) |
| Cotrimoxazole | 18 (25.71) | 25 (32.89) | 21 (25.30) |
| Piperacillin/tazobactam | 19 (27.14) | 16 (21.05) | 10 (12.04) |
Frequency of Antibiotic Resistance
This study analyzed bacterial infections across three hospitals based on culture results, identifying 222 confirmed cases. The most prevalent bacteria were Streptococcus pneumoniae (24.77%), Methicillin-Resistant Staphylococcus aureus (MRSA) (20.81%), Klebsiellapneumoniae (16.67%), Escherichia coli (13.86%), Pseudomonas aeruginosa (8.11%), Salmonella (10.36%), and Shigella (5.41%), respectively (Table 4).
| Culture Results | Golestan (N = 70) | Imam Khomeini Hospital (N = 76) | Abuzar Hospital (N = 83) | Total (229) |
|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus | 10 (14.29) | 17 (22.37) | 19 (22.89) | 46 (20.81) |
| Streptococcus pneumoniae | 17 (24.29) | 22 (28.95) | 16 (19.28) | 55 (24.77) |
| Klebsiella | 14 (20.00) | 10 (13.16) | 13 (15.66) | 37 (16.67 ) |
| Escherichia coli | 6 (8.57) | 8 (10.53) | 17 (20.49) | 31 (13.86 ) |
| Pseudomonas aeruginosa | 3 (4.29) | 8 (10.53) | 7 (8.43) | 18 (8.11 ) |
| Salmonella | 9 (12.86) | 6 (7.89) | 8 (9.64) | 23 (10.36) |
| Shigella | 5 (7.14) | 5 (6.58) | 2 (2.41) | 12 (5.41) |
Bacterial Frequency Based on Culture Results a
5. Discussion
For over fifty years, antibiotics have been used for the rapid and effective treatment of infections. Throughout this time, there have been many changes in the types of antibiotics available and in the sensitivity and resistance of bacteria to them. Numerous studies worldwide have been initiated to investigate the stability of microbes against antibiotics and the inefficacy of new antibiotics. The primary factor contributing to antibiotic resistance is natural selection (3, 4). This study was conducted by gathering data from three major hospitals in Ahvaz. The most commonly used antibiotics for both children and adults were third-generation cephalosporins, while quinolones and penicillins were the least utilized antibiotics.
The prevalence of antibacterial use in Golestan and Imam Khomeini Hospitals, at 43.2% and 44.1%, respectively, is consistent with findings from similar studies indicating high rates of antibiotic prescriptions (14, 15). Notably, Abuzar Hospital reported even higher usage rates, particularly in the PICU (86.1%), suggesting a critical need for effective antimicrobial treatment in severely ill children (16). However, these figures contrast with studies in developed countries, where antibiotic prescribing rates are often lower due to stringent stewardship programs. Overprescription in the pediatric population can lead to significant issues, such as antibiotic resistance and alterations in the microbiome, emphasizing the importance of implementing targeted stewardship initiatives to optimize antibiotic use and enhance patient safety (17). Addressing this issue is crucial, as inappropriate use of antimicrobials can have lasting impacts on children's health and the efficacy of available treatments (14).
In the analysis of antibiotic usage patterns at Golestan, Imam Khomeini, and Abuzar Hospitals, beta-lactams, including first- to fourth-generation cephalosporins and carbapenems, were found to be the most commonly used antibiotics, comprising 43%, 42%, and 46% of total prescriptions, respectively. Among these, third-generation cephalosporins accounted for 28.6%, 20%, and 32.4% of antibiotic use across the hospitals. This high reliance on beta-lactams aligns with findings from other studies that demonstrate their effectiveness against prevalent pathogens in clinical settings (18, 19). However, the emergence of multidrug-resistant strains, such as carbapenem-resistant Klebsiella pneumoniae and multidrug-resistant Shigella species, is a growing concern, as these pathogens harbor extended-spectrum beta-lactamase (ESBL) genes, significantly complicating treatment options (20). This aspect highlights a potential contradiction with the current practice observed in these hospitals; while beta-lactams are frequently prescribed, increasing resistance patterns challenge their utility as first-line treatments (18). Thus, the significant reliance on beta-lactams underscores the urgent need for antibiotic stewardship programs to address these resistance issues and optimize treatment efficacy.
The findings regarding antimicrobial resistance in Golestan, Imam Khomeini, and Abuzar Hospitals indicate alarmingly high resistance rates for cefazolin (81.42%), ampicillin (93.77%), and piperacillin (94.02%). These statistics highlight a significant challenge in treating infections caused by common pathogens in these healthcare settings. The elevated resistance rates for beta-lactam antibiotics, such as cefazolin and ampicillin, are consistent with previous research indicating widespread resistance mechanisms, including the distribution of resistance genes among isolates, such as those found in MRSA strains (21, 22). In contrast, the lowest rates of resistance were observed for ciprofloxacin (18.57%) and piperacillin/tazobactam (12.04%), suggesting that these agents may still maintain effectiveness against certain resistant pathogens. Notably, the emergence of plasmid-mediated quinolone resistance genes in P. aeruginosa further complicates the resistance landscape and indicates the need for vigilant antibiotic stewardship and surveillance programs, as these resistant strains pose significant risks in clinical environments (23). Overall, the disparity in resistance rates emphasizes the necessity for targeted interventions to mitigate the impact of antimicrobial resistance and preserve the efficacy of available treatment options (22).
Soltani et al. (5)discovered that in the pediatric ward, ceftriaxone (29.2%) and vancomycin (15%) were the most commonly used antibiotics, while in the neonatal ward, ampicillin (34.7%) and cefotaxime (14.7%) were prevalent. Another study revealed that cephalosporins were most commonly used in children, while penicillins and aminoglycosides were popular in infants (24). A meta-analysis investigating the resistance of antibacterial agents to E. coli revealed the following antibiotic resistance rates: Clarithromycin 21%, metronidazole 62%, clarithromycin and metronidazole 16%, ciprofloxacin 24%, levofloxacin 18%, erythromycin 29%, furazolidone 13%, tetracycline 8%, and amoxicillin 15%. Additionally, furazolidone and the combination of clarithromycin/metronidazole exhibited higher resistance levels in children (25).
In contrast, Tabatabaei et al. studied antibiotic resistance in children with UTIs in Iran and found high resistance rates for cotrimoxazole (97%), nalidixic acid (93%), cefotaxime (77%), and amoxicillin (62%) (26). Another study reported a 12.1% resistance rate for azithromycin in adults, with sepsis being most common in children and skin and soft tissue infections being prevalent in adults (27). The findings from Golestan and Imam Khomeini Hospitals highlight the prevalence of skin and soft tissue infections and sepsis as the most common diagnoses requiring antimicrobial treatment. This aligns with existing literature, which emphasizes the significant burden of these infections in hospital settings (28, 29), often attributed to increased rates of comorbidities and access to healthcare services. In contrast, at Abuzar Pediatric Hospital, pneumonia and lower UTIs were most prevalent, accounting for 35.1% and 21.1% of cases, respectively. This variation may reflect differences in patient demographics and the clinical focus of pediatrics compared to adult care institutions. Furthermore, the high incidence of pneumonia emphasizes the need for effective vaccination and prevention strategies in young populations to reduce the burden of respiratory infections. Overall, these results underscore the necessity for targeted antimicrobial stewardship and infection control measures tailored to the specific needs of each patient population (30).
The data regarding the frequency of single-dose surgical prophylaxis in Golestan and Imam Khomeini Hospitals reveals notable disparities between adults and children, with rates of 1% and 20%, respectively. This significant difference highlights a potential gap in preventive strategies, particularly in adult surgical practices. The absence of prophylaxis for certain infections, such as upper UTIs, plastic surgeries, orthopedic surgeries, gastrointestinal infections, and central nervous system infections, in both adult and pediatric populations indicates a need for greater awareness and adherence to established guidelines on surgical prophylaxis. Specifically, the low prophylaxis rate in the obstetrics and gynecology ward at Imam Khomeini Hospital (5%) and the 2% rate for children’s surgery in Abuzar Hospital suggest an opportunity for improvement in these areas. A study in Italy showed that infants received the most antibiotics for the prophylaxis of prematurity and sepsis, with 30.2% of antibiotic prescriptions for sepsis treatment. In older children, 64.4% of antibiotics were used for infection treatment, and 35.5% were used for prophylaxis (24).
Another study showed that infectious diseases, including UTIs (20.8%), bone and joint infections (20.4%), chronic wound skin infections (13.4%), soft tissue abscesses (13%), and gastroenteritis (10.8%), were the main reasons for antibiotic use (3). The analysis of bacterial infections across three hospitals, which confirmed 222 cases, reveals significant insights into the microbial landscape affecting patient populations. The predominance of Streptococcus pneumoniae (24.77%) and MRSA (20.81%) underscores the challenge of dealing with resistant pathogens, highlighting an urgent need for effective infection control measures and antimicrobial stewardship. Other notable pathogens, such as K. pneumoniae (16.67%) and E. coli (13.86%), further indicate the complexity of infections commonly seen in clinical settings, which can contribute to increased morbidity and healthcare costs. These findings align with global trends of rising antibiotic resistance, particularly among hospital-associated infections (13). Furthermore, the isolation of P. aeruginosa, Salmonella, and Shigella emphasizes the importance of vigilant surveillance and targeted therapeutic strategies, as these bacteria are often linked to severe infections and outbreaks. Overall, the study highlights the necessity for ongoing monitoring of bacterial infections to inform treatment protocols and prevention strategies in hospital settings (5).
This study had several limitations, including the impact of the COVID-19 pandemic (31) and the characteristics of the statistical population. A study with a longer duration and a more robust surveillance system is needed for continuous monitoring of antibiotic resistance. The information related to antibiotic resistance was limited due to the small number of positive cultures, which may have led to biased conclusions. It is recommended to establish specific guidelines for prescribing antibiotics for preoperative prophylaxis and post-surgical infections. Additionally, committees to monitor antibiotic use should be formed in every hospital.
5.1. Conclusions
A high level of bacterial resistance was observed for ampicillin and cefazolin antibiotics. Ceftriaxone was the most commonly used antibiotic for both children and adults. Continuous antimicrobial surveillance is essential to combat antibiotic resistance in local, provincial, and national referral hospitals. Effective strategies must be implemented to reduce the misuse of available antibiotics. A national surveillance program is necessary to monitor resistance rates, promote the rational use of antimicrobial drugs, and implement measures to improve patient management outcomes.