1. Background
On March 11, 2020, SARS-CoV-2 triggered a pandemic known as COVID-19, which was first identified in Wuhan, China, approximately two months earlier. Subsequently, fungal co-infections such as aspergillosis, candidiasis, and mucormycosis began to appear, especially in intensive care patients. Mucormycosis, in particular, was increasingly detected during the pandemic, with the initial case of COVID-19-associated mucormycosis (CAM) reported in Chile. These cases began to be increasingly reported, especially in India, in early 2021 (1). The sudden increase in mucormycosis cases during the pandemic has posed a new challenge for clinicians. The mortality rate of CAM exceeded 50% despite aggressive medical and surgical interventions (2, 3).
Mucormycosis, formerly known as zygomycosis, is a highly aggressive, invasive fungal infection affecting a diverse patient group. It is caused by fungi collectively called Mucorales, commonly associated with Cunninghamella, Rhizomucor, Apophysomyces, Rhizopus, Mucor, Lichtheimia, and Saksenaea (4). The most prevalent predisposing factors include granulocytopenia, immunosuppression, diabetes, and penetrating trauma (5). Mucormycosis, colloquially known as "black fungus," has been frequently detected among COVID-19 patients, especially in India (6). Mucormycosis cases have been sporadically reported in developing countries, primarily in patients with uncontrolled diabetes or trauma, and have shown high rates of morbidity and mortality (7). Additionally, the increase in the incidence of mucormycosis in European countries and the USA over the last two decades is attributed to the rise in solid organ transplantation, hematological malignancy, and diabetes cases (8).
2. Objectives
Our goal was to identify the clinical profiles of patients with CAM, compare them with a control group, and identify risk factors for the development of CAM.
3. Methods
Kayseri City Education and Research Hospital is a tertiary education center and regional hospital with 1,600 beds. From September 2020 to October 2022, 39 cases of CAM were recorded at Kayseri City Education and Research Hospital. Proven mucormycosis was defined as a patient with compatible clinical and radiological features and showing aseptate broad hyphae that were branching at right angles on direct microscopic examination of tissue/sterile material and showing invasive hyphae in tissues with or without positive fungal culture on histopathological examination. Cases with a positive reverse transcription-polymerase chain reaction for SARS-CoV-2 during the 60 days before mucormycosis diagnosis were considered CAM (case group) (9). COVID-19 was diagnosed by positive reverse transcription polymerase chain reaction (RT-PCR). The control group was selected blindly in a 1:2 ratio among patients who did not develop mucormycosis and were hospitalized, discharged, or died due to COVID-19, to be similar to the case group regarding age and hospitalization date. Demographic characteristics of the patients, underlying comorbidities, infection site, clinical findings of the infection, treatment given, and in-hospital mortality were recorded.
We utilized the IBM SPSS Statistics Windows edition (Release 27.0.1.0) for statistical analysis. In the statistical evaluation of the data obtained from the study, categorical data were summarized in frequency and percentage, and discrete variables were summarized in terms of mean ± standard deviation or median value (interquartile range), depending on the data distribution. After the normal distribution test of continuous variables was performed, the independent sample t-test was used for two-group comparisons for those with normal distribution, and the non-parametric Mann-Whitney U test was used for two-group comparisons for variables incompatible with normal distribution. Normality checks for continuous measurements were tested with the Shapiro-Wilk test. The level of statistical importance was accepted as 0.05. We used the multivariate binary logistic regression analysis to test potential risk factors for CAM. We developed a model that included gender, diabetes, cumulative dose of steroids (dexamethasone equivalent), duration of steroid treatment, and tocilizumab/anakinra treatment.
4. Results
In our study, 39 patients were diagnosed with CAM. The average age of the patients in our study was 66 ± 11.5 years. The average age of the CAM group was 66.9 ± 11.6 years, and the average age of the control group was 65.6 ± 11.5 years (P = 0.556). Of the patients, 54.7% (n = 64) were male, and the proportion of men was statistically significantly higher in the CAM group (74.4% vs. 44.9%, P = 0.003). The diabetes rate was 51.3% (n = 60) in all patients, and the diabetes rate was higher in the CAM group (69.2% vs. 42.3%, P = 0.006). Regarding in-hospital mortality, the mortality rate was higher in the CAM group (56.4% vs. 14.1%, P < 0.001). A group comparison of the patients in terms of comorbidities is shown in Table 1.
| Variables | Total (n = 117) | CAM (n = 39) | Controls (n = 78) | P-Value | Odds Ratio (95 % CI) |
|---|---|---|---|---|---|
| Age | 66 ± 11.5 | 66.9 ± 11.6 | 1.01 (0.9 - 1) c | ||
| Male | 64 (54.7) | 29 (74.4) | 35 (44.9) | 0.003 | 3.6 (1.5 - 8.3) |
| Diabetes | 60 (51.3) | 27 (69.2) | 33 (42.3) | 0.006 | 3.1 (1.4 - 6.9) |
| Uncontrolled diabetes | 19 (33.9) | 11 (40.7) | 8 (27.6) | 0.299 | 1.8 (0.6 - 5.5) |
| Hypertension | 73 (62.4) | 23 (59) | 50 (64.1) | 0.589 | 0.8 (0.4 - 1.8) |
| COPD | 24 (20.5) | 11 (28.2) | 13 (16.7) | 0.145 | 1.9 (0.8 - 4.9) |
| Cardiovascular disease | 35 (29.9) | 12 (30.8) | 23 (29.5) | 0.886 | 1.06 (0.5 - 2.5) |
| CKD | 12 (10.3) | 7 (17.9) | 5 (6.4) | 0.052 | 3.2 (0.9 - 10.8) |
| Solid organ malignancy | 61 (5.1) | 1 (2.6) | 5 (6.4) | 0.374 | 0.4 (0.04 - 3.4) |
| Hematological malignancy | 5 (4.3) | 3 (7.7) | 2 (2.6) | 0.196 | 3.2 (0.5 - 19.8) |
| Transplant patient | 8 (6.8) | 5 (12.8) | 3 (3.8) | 0.07 | 3.7 (0.8 - 16.3) |
| Obesity | 34 (29.1) | 12 (28.2) | 22 (30.8) | 0.773 | 1.1 (0.5- 2.6) |
| Tocilizumab | 16 (13.7) | 6 (15.4) | 10 (12.8) | 0.704 | 1.2 (0.4- 3.7) |
| Anakinra | 13 (11.1) | 1 (2.6) | 12 (15.4) | 0.038 | 0.15 (0.02 - 1.2) |
| LOS-hospital, days, median (IQR) | 13 (9 - 24.5) | 37 (24 - 40) | 10 (7.7 - 13.2) | < 0.001 d | 1.7 (1.3 - 2.1) c |
| Ward | 74 (63.2) | 9 (23.1) | 65 (83.3) | < 0.001 | 0.06 (0.02- 0.15) |
| Intensive care Unit | 43 (36.8) | 30 (76.9) | 13 (16.7) | < 0.001 | 16.6 (6.4 - 43.2) |
| Cumulative dose of Dexamethasone equivalent | 141.8 ± 75.4 | 191 ± 61.4 | 117 ± 69.8 | < 0.001 b | 1.02 (1 - 1.02) c |
| Duration of steroid administration (days) | 12 ± 6.2 | 16.5 ± 6.2 | 9.8 ± 4.7 | < 0.001 b | 1.2 (1.1 - 1.3) c |
| Mortality | 33 (28.2) | 22 (56.4) | 11 (14.1) | < 0.001 | 7.9 (3.2 - 19.4) |
Abbreviations: COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
aObesity is a Body Mass Index greater than or equal to 30.
b Independent-Samples t-test.
c Binary Logistic regression analysis.
d Mann–Whitney U test. Other P-values, pearson's chi-squared test.
e Values are expressed as No. (%) or mean ± SD.
When patients were examined regarding length of stay (LOS) in the hospital, the median was 13 days (9 - 24.5). The median LOS in the hospital for the CAM group was 37 days, while it was 10 days for the control group (P < 0.001). The rate of hospitalization in intensive care was higher in the CAM group (76.9% vs. 16.7%, P < 0.001), whereas the rate of hospitalization in the ward was higher in the control group (23.1% vs. 83.3%, P < 0.001). The cumulative steroid dose was higher in the CAM group compared to the control group (191 ± 61.4 vs. 117 ± 69.8, P < 0.001). The duration of steroid treatment was 16.5 ± 6.2 days in the CAM group, while it was 9.8 ± 4.7 days in the control group (P < 0.001) (Table 1).
In the between-group comparison, male gender was associated with a 3.6-fold (95% CI: 1.5 - 8.3) higher likelihood of CAM, and diabetes was associated with a 3.1-fold (95% CI: 1.4 - 6.9) higher likelihood of CAM. Similarly, the duration of steroid therapy was associated with a 1.2 - fold (95% CI: 1.1 - 1.3) higher odds of CAM. When the patients were examined in terms of laboratory values, the median creatine value of the patients in the CAM group was higher (1.15 vs. 0.92 mg/dL, P = 0.002). Similarly, the median values of procalcitonin (0.17 vs. 0.12 µg/L), fibrinogen (5665 vs. 5060 mg/L), and ferritin (720 vs. 502 µg/L) were found to be higher in the CAM group (P = 0.021, 0.05, and 0.008, respectively) (Table 2). A group comparison of the patients regarding laboratory values is shown in Table 2.
| Variables | Total (n = 117) | CAM (n = 39) | Controls (n = 78) | P-Value |
|---|---|---|---|---|
| WBC, (× 103/mm3 b) | 7.6 ± 3.5 | 7.7 ± 4.2 | 7.6 ± 3 | 0.796 a |
| Lymphocyte, (× 103/mm3) | 1.0 (0.6 - 1.4) | 0.7 (0.5 - 1.5) | 1.05 (0.7 - 1.4) | 0.258 |
| Haemoglobin, (g/dL b) | 13.2 ± 2.2 | 12.6 ± 2.6 | 13.4 ± 2 | 0.060 a |
| Platelets, (×103/mm3) | 180 (134 - 237) | 167 (109 - 223) | 183 (142 - 242) | 0.190 |
| Creatinine, (mg/dL) | 0.96 (0.8 - 1.24) | 1.15 (0.8 - 2.1) | 0.92 (0.7 - 1.1) | 0.002 |
| CRP, (mg/L) | 67 (33 - 135) | 68 (40 - 150) | 65 (30 -127) | 0.230 |
| Procalcitonin, (µg/L) | 0.13 (0.08 - 0.23) | 0.17 (0.1 - 0.64) | 0.12 (0.08 - 0.19) | 0.021 |
| D-dimer, (µg/L) | 430 (240 - 769) | 532 (240 - 825) | 407 (240 - 730) | 0.595 |
| Fibrinogen, (mg/L) | 5200 (4150 - 6240) | 5665 (4476 - 7538) | 5060 (4070 - 5970) | 0.05 |
| Ferritin, (µg/L) | 480 (195 - 797) | 720 (357 - 1247) | 502 (147 - 617) | 0.008 |
| Hemoglobin (A1c, % b) | 7.8 ± 2.1 | 8.1 ± 1.9 | 7.6 ± 2.2 | 0.280 a |
a Independent-Samples t-test, other P-values, Mann–Whitney U test.
b Values are expressed as mean ± SD, others values= Median (IQRs).
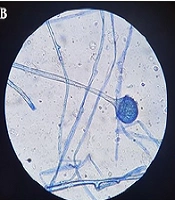
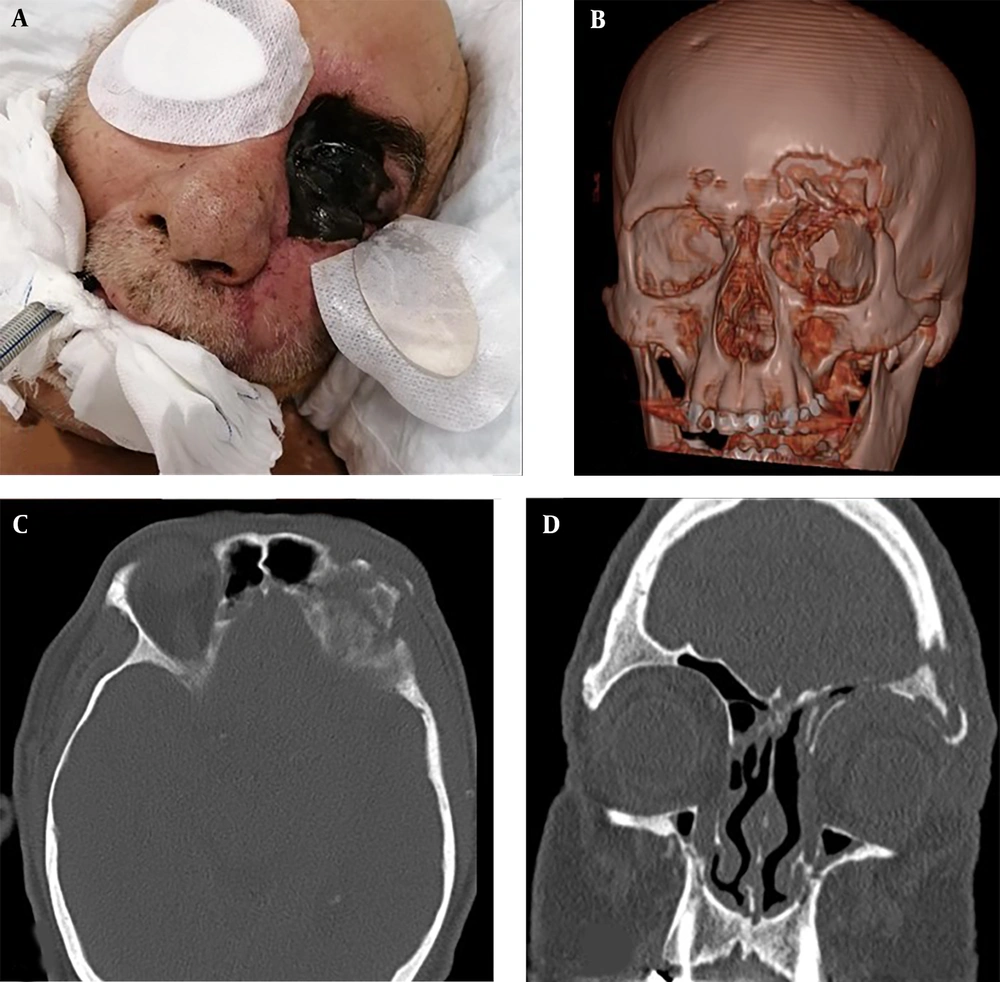
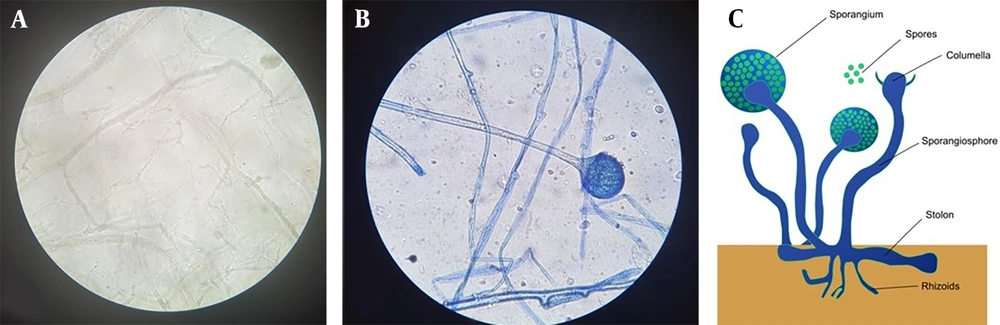
When the characteristics of the CAM group were examined, it was determined that paranasal involvement was the most common (56.4%), followed by rhino-orbital involvement (33.3%) (Figure 1 and Table 3). Microscopic and histopathological examination of biopsy materials from all patients revealed hyphae typical of Mucorales (Figure 2). Mucor was grown in the cultures of 34 (87.2%) of the patients. It was determined that 16 patients (41%) were treated with combined antifungal therapy (Liposomal Amphotericin B + Posaconazole), and 30 patients (76.9%) underwent more than one sinoscopic debridement. The most common finding in mucormycosis cases was nasal ulcer or necrosis (51.3%), followed by periorbital swelling (48.7%), proptosis (46.2%), and facial swelling (46.2%). The average time between the date of COVID-19 identification and the onset of Mucor symptoms was 21.7 ± 15.6 days.
| Variables | No. (%) |
|---|---|
| Site of disease | |
| Paranasal sinus | 22 (56.4) |
| Rhino-orbital mucormycosis | 13 (33.3) |
| Rhino-orbital-cerebral mucormycosis | 4 (10.3) |
| Pulmonary | 1 (2.6) |
| Clinical finding | |
| Nasal ulceration/ necrosis | 20 (51.3) |
| Periorbital swelling | 19 (48.7) |
| Facial swelling | 18 (46.2) |
| Proptosis | 18 (46.2) |
| Facial paralysis | 12 (30.8) |
| Treatment | |
| Liposomal amphotericin B | 20 (51.3) |
| Liposomal amphotericin B + posaconazole | 16 (41) |
| Posaconazole | 3 (7.7) |
| Sinoscopic debridement | 35 (89.7) |
| 1 debridement | 5 (12.8) |
| > 1 debridements | 30 (76.9) |
| Duration of onset of mucor symptoms from date of COVID diagnosis | 21.7 ± 15.6 days |
| Onset of symptoms (≤ 18 days) | 19 (48.7) |
| Onset of symptoms (> 18 days) | 20 (51.3) |
In binary logistic regression analysis, male gender (OR, 3.9; 95% CI, 1.4 – 11.3), diabetes mellitus (OR, 4.4; 95% CI, 1.5 – 12.4), more than ten days of steroid use (OR, 5.5; 95% CI, 1.3 – 22.4), and tocilizumab/anakinra use (OR, 0.23; 95% CI, 0.06 – 0.8) were identified as risk factors for the development of CAM (p values 0.011, 0.005, 0.019, and 0.020, respectively) (Table 4).
| Variables | Odds Ratio | 95 % CI | P-Value |
|---|---|---|---|
| Male | 3.9 | 1.4 - 11.3 | 0.011 |
| Diabetes mellitus | 4.4 | 1.5 - 12.4 | 0.005 |
| Cumulative dose (dexamethasone equivalent) | |||
| ≥ 120 (mg) | 3.3 | 0.7 - 14.7 | 0.118 |
| Duration of steroid administration | |||
| > 10 (days) | 5.5 | 1.3 - 22.4 | 0.019 |
| Tocilizumab/anakinra | 0.23 | 0.06 – 0.8 | 0.020 |
5. Discussion
Our study is the only case-control study with the highest number of CAM patients conducted in Turkey. As a regional hospital, we are a tertiary care center visited by many patients from surrounding provinces. Our aim was to identify risk factors for the development of CAM. In our study, 39 cases were diagnosed with CAM. The average age was 66 ± 11.5 years. Male gender was associated with a 3.6-fold higher odds of CAM, and diabetes was associated with a 3.1-fold higher odds of CAM. Similarly, the duration of steroid therapy was associated with a 1.2-fold higher odds of CAM. It was observed that the median creatine, procalcitonin, and fibrinogen values of the patients in the CAM group were higher. In binary logistic regression analysis, male gender, diabetes mellitus, more than ten days of steroid use, and tocilizumab/anakinra use were identified as risk factors for the development of CAM.
Mucormycosis is a significant mortal disease that affects patients of all ages, with or without immunosuppression, depending on underlying risk factors. Mucormycosis is a rare infection in children; therefore, there are few case studies in the literature. An 8-year retrospective study conducted on 164 patients in Iran determined that approximately one-quarter of the total population with mucormycosis comprised children (10). It has been stated that type 2 diabetes and widespread corticosteroid use may be responsible for the increase in mucormycosis with the COVID-19 pandemic in India. The rate of mucormycosis has increased significantly in people over 45 (11). In our study, the average age of the CAM group was 66.9 years, and there was no significant difference between them and the control group (65.6 years). A literature review published in 2022 examined clinical studies, meta-analyses, and systematic reviews to identify sex differences as a risk factor for invasive fungal diseases. The results showed a higher frequency of all fungal infections except invasive candidiasis in men (12). In our study, male gender was associated with a 3.6-fold (95% CI: 1.5 - 8.3) higher probability of CAM.
Research shows that individuals with diabetes are more susceptible to infections such as mucormycosis than healthy individuals. Diabetes is associated with high plasma glucose levels and acidic PH. In individuals with diabetes, the immune system may be weakened, increasing the risk of infection. In particular, rare diseases such as mucormycosis can lead to severe consequences in individuals with diabetes. Various factors, including a decrease in T lymphocyte count, neutrophilic dysfunction, leukocyte apoptosis, and impaired dendritic cell function, may play a role in the severe complications linked to mucormycosis in diabetic individuals (13). Our research findings reveal that diabetes significantly increases the risk of developing CAM, with an odds ratio of 4.4 (OR, 4.4; 95% CI, 1.5 – 12.4).
In our study, the in-hospital mortality rate was 56.4%. A study published in 2005 that reviewed 929 cases of mucormycosis found that the overall all-cause mortality rate was 54%. The mortality rates associated with mucormycosis in this investigation displayed variability contingent upon the patient's foundational health condition, fungal strain, and anatomical site of infection. Noteworthy distinctions in mortality rates were observed among subjects afflicted with mucormycosis, with figures at 46% for sinus involvement, 76% for pulmonary afflictions, and 96% for disseminated manifestations (4). In our study, paranasal involvement was the most common form of Mucor in more than half of the CAM patients, and rhino-orbital involvement was second.
In addition to case reports indicating that CAM increases with steroid treatment and diabetes, hyperglycemia has been stated to be associated with the development of CAM, independent of pre-existing diabetes and high steroid treatment (14, 15). In our study, diabetes was a risk factor on its own. However, more than ten days of steroid therapy rather than the cumulative dose of steroid therapy was a significant risk factor for CAM (OR, 5.5; 95% CI, 1.3 – 22.4). Hyperinflammation and cytokine storm are known to cause lung damage in COVID-19, and tocilizumab, an IL-6 receptor antagonist, has been shown to positively affect survival and respiratory function (even at low doses) (16). Similarly, anakinra is a recombinant IL-1 receptor antagonist used in COVID-19 and has been reported to reduce the progression to severe disease and the need for mechanical ventilation (17). In our study, tocilizumab/anakinra use was a negative risk factor for the development of CAM (OR, 0.23; 95% CI, 0.06 – 0.8).
The main limitation of our study is that it is retrospective and single-center. There is a need for multicenter studies in which more patients will be examined to support our findings.
5.1. Conclusions
In our study, more than half of the CAM patients died despite medical and surgical treatments. Male gender, diabetes mellitus, and steroid use for more than ten days were positive risk factors, and tocilizumab/anakinra use were negative risk factors for the development of CAM. We believe that clinicians should be more cautious about steroid therapy and its duration, as well as diabetes.