1. Background
Gut microbiota play a key role in the overall health of the host, and their interaction with the mucosal immune system maintains homeostasis in the gut. However, the complex interaction of several environmental, genetic, and microbial factors may lead to microbiota perturbation. Multiple lines of evidence suggest that in certain colon diseases, such as inflammatory bowel disease (IBD) or colorectal cancer (CRC), the intestinal microbiota differs from that of healthy individuals, indicating the role of gut dysbiosis in the pathogenesis of these diseases (1-3). Inflammatory bowel disease is a chronic, relapse-remitting disease characterized by intestinal inflammation, while CRC is known as the fourth most common cause of cancer-related death in the world. The current reliable method for diagnosing colon diseases is colonoscopy, which is invasive, uncomfortable, time-consuming, and costly (4-6).
Pathobionts are opportunistic members of the gut microbiota, whose selective enrichment as a result of intestinal dysbiosis can cause several disorders, potentially leading to life-threatening conditions (7). Some strains of Escherichia coli are pathobionts that can participate in the initiation or development of colon diseases through the induction of pro-inflammatory pathways or the production of genotoxins (1). Cyclomodulins involved in the genesis of colon diseases have been identified in the colon flora (particularly in E. coli strains) of affected patients. Various cyclomodulins, including colibactin in the pks genomic island, cytotoxic necrotizing factor (CNF), cycle inhibiting factor (CIF), and cytolethal distending toxin (CDT), are genotoxic and/or affect cell-cycle progression, proliferation, cell differentiation, and programmed cell death (1, 2). Furthermore, afa-C+ diffusely adherent E. coli (DAEC) have been linked to human colon diseases (6, 8).
Due to the limitations of colonoscopy and the strong reluctance of patients to undergo the procedure, along with the suggested role of pathobionts in colon diseases, the use of these factors as biomarkers in non-invasive diagnostic procedures has become prominent. Additionally, the potential benefits of using them in targeted therapies, instead of conventional symptom-relief-based treatments, have been proposed (6). Many attempts have been made to assess the potential of E. coli pathobionts for detecting IBD or CRC, but in most cases, biopsy specimens from patients at different stages of colon diseases have been used. Since the prevalence of these bacteria in colon diseases varies among different populations (1), the aim of this study was to clarify the association between certain E. coli pathobionts (cyclomodulin-positive and afa-C+ DAEC isolates) and colon diseases (IBD, CRC) in comparison to healthy subjects in the Iranian population.
2. Objectives
The present study differs from similar studies in two ways: Firstly, by analyzing stool samples as a non-invasive screening method, and secondly, by including patients in the early stages of CRC without any previous chemotherapy treatment. Additionally, some characteristics of the isolated E. coli were determined and compared with those from healthy subjects.
3. Methods
3.1. Bacterial Isolates
Simple random sampling was conducted to collect stool samples from individuals who underwent colonoscopy at university-affiliated hospitals or clinics in two Iranian cities, Kerman and Yazd, between 2021 - 2022. The selected patients included newly identified cases of CRC (n = 31) and IBD (n = 46). To eliminate the microbial alterations induced by colonic lavage, patients were asked to provide stool samples a few days after the colonoscopy procedure. Exclusion criteria included antibiotic treatment within the last two weeks or any previous chemotherapy treatment. The control group (n = 67) included healthy individuals without any gastrointestinal problems who were approximately matched by age and gender with the patient group. In the research laboratory of the microbiology department, stool samples were cultured on MacConkey agar (Conda, Spain), and after overnight incubation at 37°C, lactose-positive colonies were identified by conventional biochemical tests, including growth on triple sugar iron agar, Christensen's iron agar, and Simmons citrate agar (Conda, Spain), as well as indole, methyl red, and Voges-Proskauer tests (9). From each sample, five separate confirmed isolates were randomly selected and stored at -80°C in trypticase soy broth containing 20% (v/v) glycerol until use.
3.2. Phylotyping
Phylogenetic classification of all E. coli isolates (5 isolates from each stool specimen) was performed using multiplex PCR, as introduced by Clermont et al. (10).
3.3. Detection of pks Gene Cluster, Other Cyclomodulin-Encoding Genes (cif, cnf-1, cdt), and Virulence Genotyping
The pks genomic island, specifically the clbN, clbA, clbQ, and clbB genes, was screened by PCR (11, 12). Cytotoxic necrotizing factor, CIF, and CDT were investigated through PCR using the cnf-1, cif, and cdt target genes, respectively (1, 13). The afa-1 operon was used for the detection of Afa-C adhesion in DAEC (6). Furthermore, the isolates were screened for harboring other virulence-associated genes, including fimH, ompC, yfgL, nlpL, iutA, iroN, chuA, fyuA, entD, and hlyA, using specific primers as described previously (4, 14-19).
3.4. Antibiotic Susceptibility Testing
The Kirby-Bauer disk diffusion method was employed to determine the susceptibility of isolates to ciprofloxacin (5 μg), imipenem (10 μg), amikacin (30 μg), tetracycline (30 μg), ceftazidime (30 μg), trimethoprim/sulfamethoxazole combination (1.25/23.75 μg), ofloxacin (5 μg), cefixime (5 μg), cefotaxime (30 μg), ampicillin (10 μg), azithromycin (15 μg), and gentamicin (10 μg). Susceptibility breakpoints were defined according to Clinical and Laboratory Standards Institute guidelines (20). Isolates showing intermediate levels of susceptibility were classified as non-susceptible. E. coli ATCC 25922 was used as the quality control strain.
3.5. Biofilm Assay
The biofilm formation assay was carried out using the microtiter plate method, as described previously, with 2% crystal violet (21). The absorbance of stained wells was measured at 570 nm. The negative control was TSB enriched with 1% glucose, and Staphylococcus epidermidis RP62A was used as the positive control. All experiments were carried out in triplicate.
3.6. Statistical Analysis
The statistical tests were performed using the SPSS program (version 25; SPSS, Inc.). The chi-square or Fisher’s exact test was used to compare variables. A P-value of < 0.05 was considered statistically significant.
4. Results
Data analysis showed that most of the isolates (29.9% and 38.8% of isolates from control and patient groups, respectively) were non-typeable by the phylotyping method used in this study. The most frequent typeable isolates were categorized as phylogroups B2 (n = 16, 23.9%), A (n = 9, 13.4%), B1 (n = 8, 11.9%), and D (n = 3, 4.5%) in the patient group, while in the control group, phylogroup A (n = 19, 28.4%) was the most frequent type, followed by D (n = 12, 17.9%) and B1 (n = 8, 11.9%). Since all five isolates obtained from one specimen showed similar phylogroups, in the next experiments, only one isolate from each specimen was investigated. As all of the isolates were positive for all four investigated colibactin-related genes (clbA, clbB, clbN, and clbQ), in further analysis, clbN was used as a surrogate marker for the pks island. The distribution of cyclomodulin-encoding genes (clbN, cnf, cdt, cif) was evaluated in all isolates.
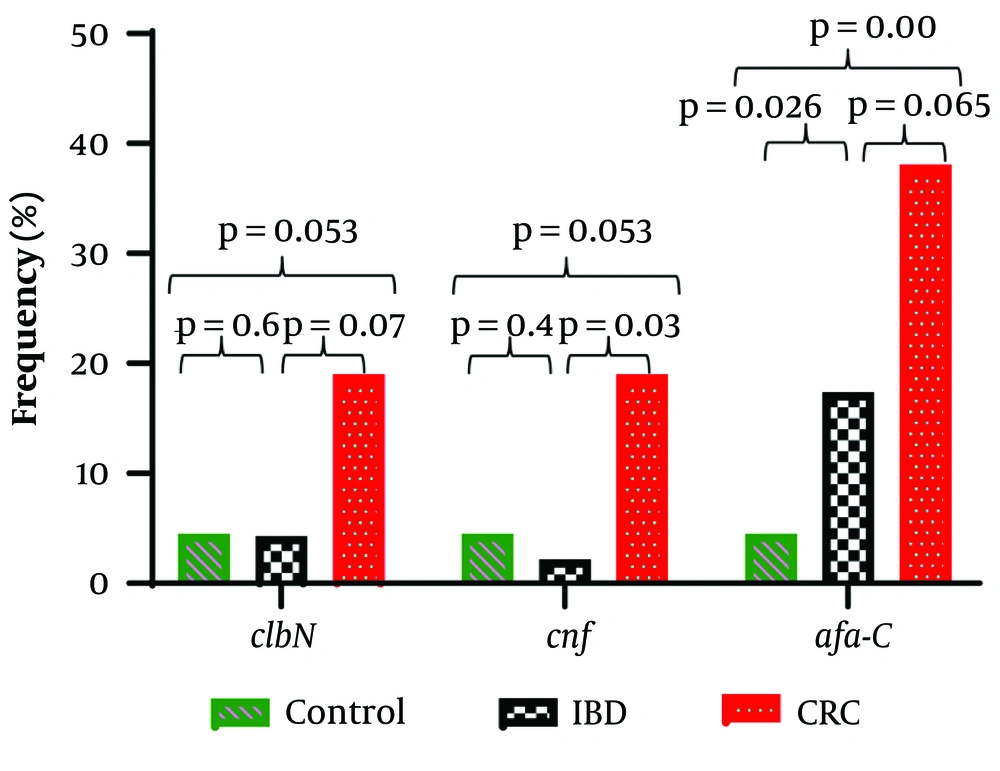
The frequencies of clbN and cnf encoding genes in the patient group were 6 (9%) and 5 (7.5%), respectively. Both of these genes were detected in only 3 (4.5%) isolates of the control group. Other investigated genes, cif and cdt, were not found in any of the studied isolates (Table 1). Overall, 5 (50%) cyclomodulin-positive isolates were obtained from CRC patients. The cnf gene was significantly higher in CRC patients compared to IBD patients (P = 0.03) and the control group (P = 0.053). The clbN gene was more prevalent in the CRC group compared to the control group, but the difference was relatively significant (P = 0.053) (Figure 1). Co-presence of two cyclomodulins (CNF and colibactin) was found in 14% of CRC patients and 4% of the control group, and the difference was significant (P = 0.017). Interestingly, 40% of cyclomodulin-harboring isolates belonged to phylogroup B2. A significant association was found between cyclomodulin positivity and phylogroup B2 (P = 0.036). A meaningful relationship was also found between afa-C+ DAEC isolates (n = 19) and placement in phylogroups B2 and A (P = 0.013).
| Genes | Total; (n = 134) | CRC; (n = 21) | IBD; (n = 46) | Control; (n = 67) | P-Value |
|---|---|---|---|---|---|
| afa-C | 19 (14.2) | 8 (38.1) | 8 (17.4) | 3 (4.5) | 0.001 |
| cnf | 8 (6) | 4 (19) | 1 (2.2) | 3 (4.5) | 0.034 |
| clbN | 9 (6.7) | 4 (19) | 2 (4.3) | 3 (4.5) | 0.068 |
Abbreviations: CRC, colorectal cancer; IBD, inflammatory bowel disease.
a Values are expressed as No. (%).
b Fisher’s exact test was used for analysis.
Iron uptake-associated genes (chuA, fyuA, iutA, and iroN) were significantly more distributed in isolates from the patient group compared to the control group and were also more prevalent in CRC patients compared to IBD patients. Regarding fimH, iutA, and chuA, significant differences were found between both CRC and IBD patients compared to the control group (P < 0.05), whereas for fyuA and iroN, a significant difference was only found between the IBD and control groups (Table 2). Additionally, iron uptake-associated genes (fyuA, iroN, and fitA) were significantly more prevalent in cyclomodulin-harboring isolates compared to the others. Regarding afa-C, 38% of CRC isolates were positive for afa-C compared to 4.5% of isolates from the control group (P = 0.001). A significant difference was also found when comparing IBD and the control group (P = 0.026). Thus, with a specificity of 95.5%, the afa-C assay detected 38% of CRCs (Table 1). The difference between CRC and IBD patients was of relatively borderline significance (P = 0.065), but the increasing frequency from controls to IBD to CRC indicates that afa-C may represent an appropriate putative marker for CRC (Figure 1).
| Genes | CRC; (n = 21) | IBD; (n = 46) | Control; (n = 67) | P-Value |
|---|---|---|---|---|
| chuA | 18 (85.7) | 31 (67.4) | 30 (44.8) | 0.001 |
| fyuA | 11 (52.4) | 32 (69.6) | 26 (38.8) | 0.006 |
| iutA | 10 (47.6) | 19 (41.3) | 12 (17.9) | 0.005 |
| iroN | 3 (14.3) | 7 (15.2) | 2 (3) | 0.02 c |
| fitA | 2 (9.5) | 2 (4.3) | 5 (7.5) | 0.64 c |
| entD | 17 (81) | 28 (60.9) | 39 (58.2) | 0.16 |
| ompC | 21 (100) | 46 (100) | 67 (100) | NA |
| yfgL | 21 (100) | 43 (93.5) | 65 (97) | 0.46 c |
| nlpL | 0 | 1 (2.2) | 0 | 0.5 c |
| eefC | 5 (23.8) | 3 (6.5) | 7 (10.4) | 0.13 c |
| fimH | 18 (85.7) | 39 (84.8) | 67 (100) | 0.001 c |
| hlyA | 0 | 1 (2.2) | 0 | 0.5 c |
Abbreviations: NA, not applicable; CRC, colorectal cancer; IBD, inflammatory bowel disease.
a Values are expressed as No. (%).
b χ2 test was used for analysis.
c Fisher’s exact test.
Regarding antibiotic resistance; except for ofloxacin (34% of isolates from patients vs. 9% from the control group; P = 0.000), no significant differences were found in antibiotic susceptibility between isolates from different groups. In terms of biofilm formation; most of the isolates (75%) were not biofilm producers, and there were no differences between the two groups regarding the ability to form biofilms (Table 3).
| Biofilm | Control; (n = 67) | IBD; (n = 46) | CRC; (n = 21) | P-Value |
|---|---|---|---|---|
| No biofilm | 50 (74.6) | 36 (78.3) | 15 (71.4) | 0.5 |
| Weak | 10 (14.9) | 6 (13) | 5 (23.8) | |
| Moderate | 3 (4.5) | 0 | 1 (4.8) | |
| Strong | 4 (6) | 4 (8.7) | 0 |
Abbreviations: CRC, colorectal cancer; IBD, inflammatory bowel disease.
a Values are expressed as No. (%).
5. Discussion
In our study despite using the new phylotyping method developed by Clermont et al. (10) and similar to another study from our region (Kerman, Iran) (22) a number of strains were not assigned to a phylogroup. This is not surprising due to the highly variable gene content in E. coli resulting from frequent gene loss and gain (10). Furthermore the existence of very rare phylotypes is conceivable but given the high frequency of these strains in the present study the first hypothesis seems more acceptable. This study showed that the most frequent E. coli strains from patients with colon diseases belonged to phylogroup B2 which is in agreement with some other studies (1, 5, 21). Phylogenetic groups of E. coli have been developed based on the acquisition of virulence factors (2). Escherichia coli strains from phylogroups B2 and D carry the heme iron acquisition gene (chuA); which plays an important role in bacterial survival within macrophages.
More importantly, by stimulating TNF-α release; chuA may promote intestinal dysbiosis involved in colon diseases (5). Similar to our results, phylogroups B2 and A were abundantly distributed among isolates from CRC and IBD patients in Zarei et al.'s study (21). Therefore, an association between IBD and carcinogenesis could be hypothesized (23). Unexpectedly phylogroup D was neither abundant in CRC patients nor in IBD patients whereas it was the second most frequent type in the control group. This finding is similar to the study by Hashemizadeh et al., conducted previously in our region (Kerman; Iran), which reported phylogroups B2 and D as more frequent than A in E. coli from healthy people (24). In contrast in another study from Iran, phylogroup D was reported as the most prevalent phylotype next to phylogroup B2 in IBD patients (5, 25).
Although a low frequency of cyclomodulin-positive isolates was found, cnf and pks were higher in isolates from CRC patients compared to the control group (P = 0.053). Colibactin encoded by the pks genomic island is a genotoxin that causes DNA double-strand breaks and instability in human eukaryotic cells (1, 2). In agreement with the present study; Iyadorai et al. from Malaysia reported pks+ E. coli in 16.7% of CRC patients and 4.3% of the control group (vs. 19% and 4.5%; respectively; in this study) (26). Similarly a low prevalence of the clb gene (1.5%) has been recently reported among E. coli isolated from students' stool samples (27). Additionally a molecular study from Sweden found a significant difference in the presence of clbA+ bacteria in the stool of CRC patients (56%) compared to the control group (18%) (6). The high prevalence may be related to the inclusion of CRC patients at different stages in this study (6). This issue has also been mentioned by Oliero et al. noting that patients in the early stages of CRC were significantly less colonized with pks+ isolates (20%) compared to patients in the late stages (52%) (3).
Cytotoxic necrotizing factor is considered a cyclomodulin that affects cytoskeletal rearrangement and cell division. The association between CNF in uropathogenic E. coli and bladder cancer has been reported (28). However; its presence in CRC patients has also been noted (1). Similar to a study from Mexico which identified the cnf gene in 17.6% of CRC patients in our study 19% of isolates from CRC patients carried this gene (1). In contrast to a prevalence of 4.5% in this study; Johnson and Stell (13) detected colibactin genes and cnf in 32% and 13% of fecal isolates from the control group respectively. DAEC isolates harbor important fimbrial (Dr) and afimbrial (Afa) adhesions which allow them to adhere strongly to enterocytes and induce cell damage even causing cell transition from epithelial to mesenchymal form (1, 29).
Although controversial; the association of DAEC with IBD and CRC has been suggested in some studies (5, 29); which is in agreement with our findings. afa-C+ DAEC were significantly more frequent in patients compared to healthy individuals with a higher prevalence in CRC patients than in IBD patients and the control group. Due to the low sensitivity of the afa-C assay for CRC diagnosis (38%) it may be used as a screening marker for the primary diagnosis of high-risk patients. The presence of afa-C+ DAEC in IBD patients may be explained by their potential to damage DNA during the early stages of colon diseases (1). In any case follow-up of these subjects might provide valuable information about the specificity of the test.
Virulence factors can be considered markers for cancer progression. According to a metagenomic study there is an enrichment of virulence factors in the microbiome of CRC patients but their abundance differs across the different stages of CRC (23). For example colibactin and siderophores are significantly more prevalent in advanced stages of CRC whereas adhesions are more prevalent in the early stages of CRC (23). Similar to Hashemizadeh et al. who reported fimH in 94% of E. coli from healthy individuals (24) it was found in all isolates from the control group in our study. However in contrast to fimH (an adhesion factor) the factors involved in iron acquisition (chuA, iutA, fyuA, and iroN) were more prevalent in patients.
5.1. Limitations
Since CRC patients in our study were newly diagnosed cases, the high prevalence of such factors in these isolates may be regarded as a warning for disease progression. Follow-up of these patients might clarify this issue, but it was a limitation of this study.
Overall the prevalence of antibiotic resistance in both studied groups (patients and healthy subjects) was higher than in some other studies (25, 30). However; there were some similarities, the frequency of resistance against ciprofloxacin in the CRC group (35%) was approximately similar to the reports of Mahmoudi et al. (35%) (30) and Aibinu et al. (21.4%) (31). The highest susceptibility was observed against amikacin and imipenem; consistent with a previous report from Iran (30). It has been confirmed that biofilm production in some intestinal microflora can be a natural process (11, 21). In agreement with the report of Zarei et al. (21) the highest frequency of biofilm formation was observed in isolates belonging to phylogroups B2 and A. No significant difference was found between the different groups regarding biofilm formation. However while Zarei et al. mostly detected weak or moderate biofilms in isolates (21) in our study weak biofilm formation or lack of biofilm formation was dominant.
Discrepancies observed between the findings of this study and those of other studies may be due to differences in the type of specimen (biopsy vs. fecal samples) diagnostic methods (culture vs. molecular methods) and stages of disease (inclusion of patients at different stages of the disease vs. early stages). Additionally although stool samples in this study were taken a few days after colonic lavage to minimize its adverse effect on microbiota, the potential role of colonic lavage in microbial alteration cannot be ignored. Other limitations of this study include the small sample size, failure to follow up patients and the use of a limited number of centers for the diagnosis of IBD and CRC patients; all of which may have influenced the results.
5.2. Conclusions
In conclusion; this study provides preliminary data on the status of certain important E. coli pathobionts involved in colon diseases. It appears that >afa-C+ DAEC was more associated with colon diseases, suggesting it may be proposed as a putative marker. However due to the limitations of the study a definitive conclusion requires more comprehensive investigations.