1. Background
Even after the SARS-CoV-2 pandemic, the risks of new outbreaks persist, particularly with the emergence of novel variants. Oxygen is essential for respiration and critical metabolic processes, depending on a continuous supply through oxidative metabolism (1). With age, decreased blood oxygen levels lead to tissue oxygen deficiency. Studies indicate that oxygen-enriched water reduces nitrate levels, improving water quality and benefiting health, especially in children (2). Oxygen molecules attach to water; when this water interacts with mitochondrial tissue, membranes split, supplying cells with oxygen. The partial pressure of oxygen (PO2) is a critical factor in assessing the relationship between oxygen-rich water and dissolved oxygen in the blood. Dissolved oxygen levels in hot water are lower than in cold, with Iranian Standard No. 6694 allowing 50 - 65 mg/L in oxygen-rich water (3).
In oral oxygen therapy (OOT), oxygen enters the circulatory system through the digestive system and portal vein. Absorption begins in the oral cavity and gastrointestinal tract, increasing blood oxygen levels as it circulates through the liver and body. This method relies on the oral and digestive systems rather than the lungs (2). Topical OOT promotes healing by enhancing angiogenesis, removing toxins, stimulating collagen synthesis, fostering new blood cell formation, increasing stem cell production, and strengthening bacterial defense mechanisms (4, 5). Using oxygen-enriched liquid can support lung function during acute respiratory diseases or obstruction. Consumption of oxygen-rich water enhances respiratory function as alveoli absorb dissolved oxygen, resulting in improved oxidation and a significant increase in blood oxygen levels within five minutes (2). Given the absence of specific antiviral treatments for severe SARS-CoV-2 respiratory disease, and recognizing hypoxia as a leading cause of death in affected patients, oxygen-rich water—an affordable option—may serve as an effective symptomatic treatment.
2. Objectives
This study investigates the potential role of oxygen-rich water in improving clinical outcomes for patients with SARS-CoV-2.
3. Methods
This double-blind, randomized controlled trial, registered under IR.AJUMS.REC.1399.658 and IRCT20201125049486N1, included 44 hospitalized patients with severe COVID-19 at Razi Hospital, Ahvaz, Iran, who were randomly selected after providing informed consent. Inclusion criteria required blood oxygen saturation below normal levels, while exclusion criteria included reluctance to cooperate or inability to drink water. After matching for demographics and underlying health conditions, patients were divided into two groups of 22 each: A control group and an intervention group.
The intervention group received 300 mL of oxygen-rich water every four hours (8 AM, 12 PM, 4 PM, 8 PM, and 12 AM) daily in standardized PET bottles, alongside standard treatment according to national guidelines. The control group received 300 mL of purified drinking water at the same intervals, also in standardized bottles, in addition to standard treatment. Blood oxygen saturation and respiratory rates were measured before and five minutes after water consumption, continuing for one week. Patients discharged prior to completing the study period were monitored via phone after receiving the required water and consulting with an infectious disease specialist. Hospitalization duration and improvement rates were recorded.
The results were analyzed using SPSS software version 24. The t-test was applied to assess age and hospitalization duration, the chi-square test was used for sex, occupation, and mortality rates, and the analysis of covariance was employed for oxygen saturation and respiratory rates.
4. Results
The demographic characteristics of the patients are detailed in Table 1. Results are presented as mean ± SD for age and as number (%) for sex. Data were analyzed using the t-test for age and the chi-Square test for sex. P-values below 0.05 were considered significant. No significant difference was found between the intervention and control groups regarding sex (P > 0.5) or occupation (P > 0.5). However, a significant difference in age was observed (P = 0.028), with the intervention group having a mean age of 49 ± 11.77 years and the control group having a mean age of 57.36 ± 12.21 years.
| Variables | Control (N = 22) | Intervention (N = 22) | P-Value b |
|---|---|---|---|
| Age | 57.36 ± 12.21 | 49.00 ± 11.77 | 0.028 |
| Gender | 0.542 | ||
| Female | 9 (40.9) | 7 (31.8) | |
| Male | 13 (59.1) | 15 (68.2) |
a Values are expressed as mean ± SD or No. (%).
b P < 0.05 was considered statistically significant.
Table 2 displays the patients' underlying conditions. Results are presented as number (%) and analyzed using the chi-Square test, with significance defined as P-values less than 0.05. No significant differences were observed between the intervention and control groups regarding any of the diseases. Consequently, analysis of covariance was utilized to compare blood oxygen saturation and respiratory rates per minute between the groups, effectively controlling for the significant age differences.
| Diseases | Control | Intervention | P-Value b |
|---|---|---|---|
| Underlying conditions | 13 (59.1) | 11 (47.8) | 0.554 |
| Diabetes | 7 (31.8) | 7 (30.4) | 1.000 |
| Blood hypertension | 7 (31.8) | 3 (13) | 0.165 |
| Cardiovascular diseases | 4 (18.2) | 2 (8.7) | 0.414 |
| Lung diseases | 2 (9.1) | 3 (13) | 1.000 |
| Hypothyroidism | 1 (4.5) | 0 (0) | 0.489 |
| Hyperthyroidism | 0 (0) | 1 (4.3) | 1.000 |
a Values are expressed as No. (%).
b P < 0.05 was considered statistically significant.
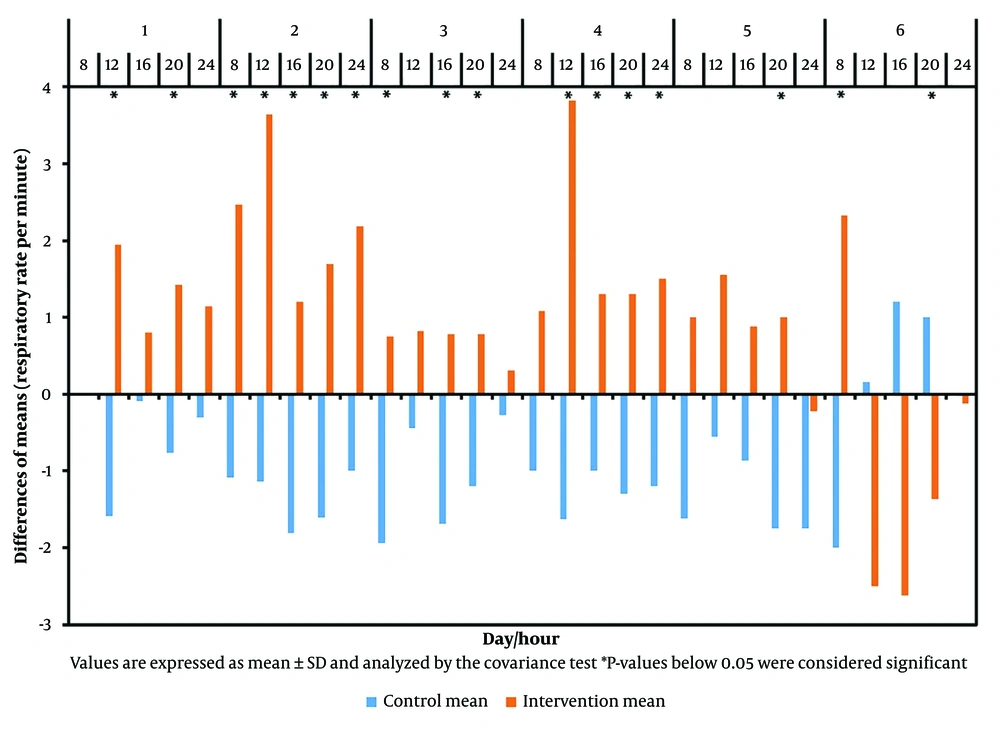
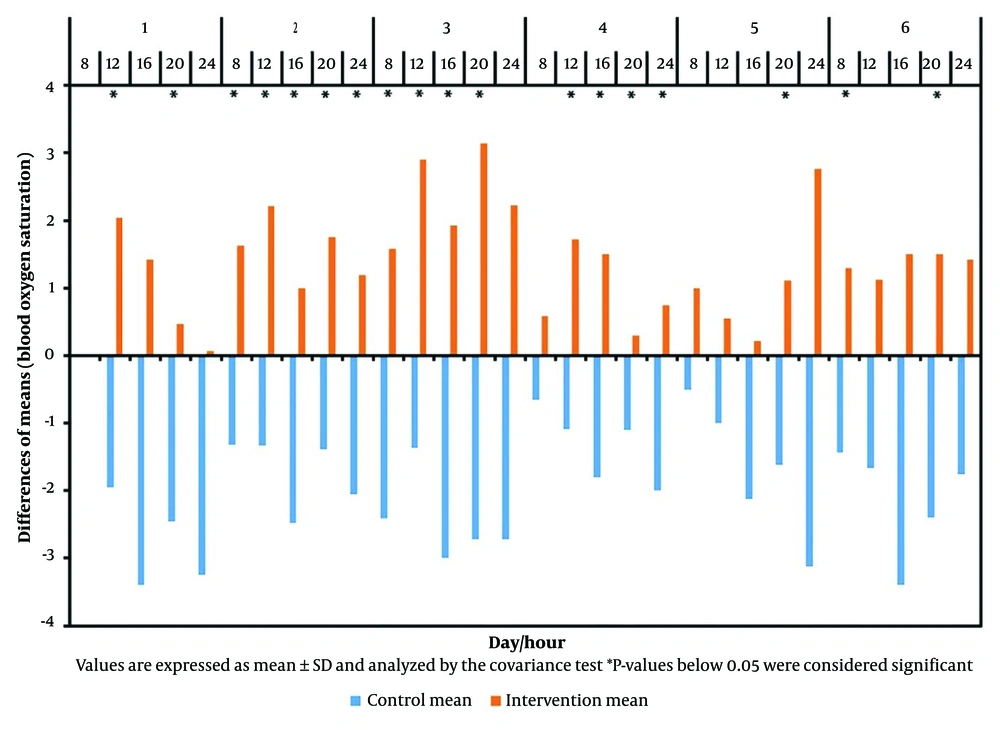
Table 3, Figure 1, and Figure 2 illustrate the differences in the mean values of blood oxygen saturation and respiratory rate per minute of patients, measured before and five minutes after consuming oxygen-rich water throughout the trial.
| Variables (d) | Time (h) | Respiratory Rate per Minute | Blood Oxygen Saturation | ||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | P-Value b | Control | Intervention | P-Value b | ||
| 1 | 12 | -1.95 ± 2.72 | 1.95 ± 2.27 | < 0.001 | -1.95 ± 3.13 | 2.04 ± 2.08 | < 0.001 |
| 16 | -0.09 ± 1.74 | 0.80 ± 3.04 | 0.284 | -3.40 ± 4.09 | 1.42 ± 3.6 | < 0.001 | |
| 20 | -0.77 ± 2.67 | 1.42 ± 2.80 | 0.017 | -2.45 ± 3.06 | 0.47 ± 4.02 | 0.031 | |
| 24 | -0.30 ± 1.84 | 1.14 ± 3.2 | 0.706 | -3.25 ± 4.6 | 0.066 ± 3.52 | 0.003 | |
| 2 | 8 | -1.09 ± 2.82 | 2.47 ± 3.09 | < 0.001 | -1.32 ± 2.66 | 1.63 ± 1.83 | 0.001 |
| 12 | -1.14 ± 2.43 | 3.64 ± 2.37 | < 0.001 | -1.33 ± 2.49 | 2.21 ± 2.63 | 0.001 | |
| 16 | -1.84 ± 2.03 | 1.20 ± 2.90 | 0.004 | -2.48 ± 3.50 | 1.00 ± 2.48 | 0.002 | |
| 20 | -1.61 ± 3.2 | 1.69 ± 1.49 | 0.001 | -1.39 ± 2.38 | 1.75 ± 2.90 | 0.003 | |
| 24 | -1.00 ± 1.45 | 2.19 ± 1.97 | < 0.001 | -2.05 ± 3.92 | 1.19 ± 3.90 | 0.025 | |
| 3 | 8 | -1.94 ± 1.75 | 0.75 ± 2.66 | 0.002 | -2.41 ± 2.37 | 1.58 ± 2.39 | 0.001 > |
| 12 | -0.44 ± 2.19 | 0.82 ± 2.92 | 0.078 | -1.37 ± 2.98 | 2.90 ± 2.54 | 0.001 > | |
| 16 | -1.69 ± 1.84 | 0.78 ± 2.04 | 0.011 | -3.00 ± 1.82 | 1.93 ± 4.35 | 0.008 | |
| 20 | -1.20 ± 1.23 | 0.78 ± 2.08 | 0.010 | -2.72 ± 1.67 | 3.14 ± 3.39 | 0.001 > | |
| 24 | -0.27 ± 3.52 | 0.307 ± 2.62 | 0.43 | -2.72 ± 1.55 | 2.23 ± 3.32 | 0.002 | |
| 4 | 8 | -1.00 ± 2.73 | 1.08 ± 4.01 | 0.189 | -0.66 ± 3.31 | 0.58 ± 1.73 | 0.062 |
| 12 | -1.63 ± 2.41 | 3.82 ± 3.15 | 0.002 | 1.09 ± 1.64 | 1.72 ± 2.00 | 0.026 | |
| 16 | -1.00 ± 1.69 | 1.30 ± 0.95 | 0.010 | -1.80 ± 2.15 | 1.50 ± 2.50 | 0.008 | |
| 20 | -1.30 ± 1.16 | 1.30 ± 1.83 | 0.002 | -1.1 ± 3.38 | 0.3 ± 3.77 | 0.140 | |
| 24 | -1.20 ± 1.55 | 1.50 ± 1.69 | 0.003 | -2.00 ± 1.33 | 0.75 ± 4.43 | 0.080 | |
| 5 | 8 | -1.62 ± 1.99 | 1.00 ± 3.89 | 0.129 | -0.50 ± 1.77 | 1.00 ± 2.26 | 0.141 |
| 12 | -0.55 ± 2.29 | 1.55 ± 2.29 | 0.337 | -1.00 ± 0.86 | 0.55 ± 2.50 | 0.219 | |
| 16 | -0.87 ± 1.72 | 0.88 ± 2.71 | 0.351 | -2.12 ± 1.88 | -0.22 ± 4.26 | 0.097 | |
| 20 | -1.75 ± 1.58 | 1.00 ± 1.86 | 0.033 | -1.62 ± 2.13 | 1.11 ± 2.20 | 0.010 | |
| 24 | -1.75 ± 1.58 | -0.22 ± 3.34 | 0. 461 | -3.12 ± 2.23 | 2.77 ± 2.33 | 0.004 | |
| 6 | 8 | -2.00 ± 1.15 | 2.33 ± 2.00 | 0.017 | -1.43 ± 1.27 | 1.30 ± 0.95 | 0.004 |
| 12 | 0.16 ± 2.22 | -2.50 ± 2.07 | 0.302 | -1.66 ± 2.65 | 1.12 ± 1.45 | 0.185 | |
| 16 | 1.20 ± 1.09 | -2.62 ± 4.06 | 0.307 | -3.40 ± 1.51 | 1.50 ± 4.53 | 0.190 | |
| 20 | 1.00 ± 0.71 | -1.37 ± 2.13 | 0.026 | -2.40 ± 1.14 | 1.50 ± 1.19 | 0.002 | |
| 24 | 0.00 ± 0.81 | -0.12 ± 1.75 | 0.221 | -1.75 ± 1.50 | 1.42 ± 3.15 | 0.099 | |
a Values are expressed as mean ± SD and analyzed by the covariance test.
b P < 0.05 was considered statistically significant.
The difference between the mean values of respiratory rate per minute of patients in both groups before and five minutes after consuming oxygen-rich water during the trial; Values are expressed as Mean ± SD and analyzed by the Covariance test. * P-values below 0.05 were considered significant.
According to Table 3, Figure 1, and Figure 2, at 12 o'clock on the first day, a significant difference in respiratory rate per minute was observed between the groups (P < 0.001). The intervention group experienced an average decrease of 1.95 units after consuming oxygen-rich water, while the control group showed an average increase of 1.59 units, resulting in a total difference of approximately 3.54 units. Concurrently, blood oxygen saturation in the intervention group increased by an average of 2.05 units, whereas it decreased by 1.95 units in the control group, leading to a mean difference of 4 units (P < 0.001).
5. Discussion
This study demonstrates that oxygen-rich water can increase blood oxygen saturation and reduce respiratory rate. Specifically, 82.75% of the intervention group experienced decreased respiratory rates, while 86.2% of the control group saw an increase. The difference in respiratory rates between the groups was significant in 58.62% of cases. Blood oxygen saturation increased in 96.55% of the intervention group and decreased in 96.55% of the control group, with a significant difference in 68.96% of cases.
Clinical reports in Russian journals have suggested positive effects of oxygenated water on patients with conditions such as morbid obesity, cholecystitis, and portal hypertension; however, these reports lack rigorous study criteria, raising concerns about their validity (6). Gelman et al. demonstrated in two studies that using oxygen in the intestinal tract improves hepatic blood circulation and overall oxygen supply, although not specifically with oxygenated water (7, 8).
Mazurok et al. found that enteral insufflation of low volumes of oxygen increased systemic oxygenation in patients with acute respiratory distress syndrome, indicating the need for further research to elucidate the mechanisms involved (9). Forth and Adam showed that intragastric oxygenated water raised oxygen levels in the abdominal cavity and portal vein of rabbits (10). Izawa et al. reported a stronger correlation between SpO2 and pulse rate in the oxygenated water group compared to the normal water group. While a significantly smaller decrease in SpO2 during walking may be beneficial, the oxygen group also exhibited a concerning increase in pulse rate (11).
A key question is whether consuming oxygenated water increases oxygen radical production. Schoenberg et al., in a study of 66 volunteers, found that drinking oxygenated water (60 mg O2/L H2O) caused a temporary, moderate rise in oxygen radicals. However, long-term consumption mitigated this effect (12), disproving the hypothesis that oxygenated water might act as an antioxidant or enhance the immune system (6).
In a study by Gruber et al. involving 24 volunteers divided into two groups, one consuming oxygenated water (190.6 ± 5.0 mg O2/L) and the other consuming normal water (6.4 ± 2.0 mg O2/L), no significant differences in laboratory parameters were observed between treatment times. Long-term consumption of oxygenated water showed no apparent harmful effects on the liver, blood, or immune system (13). Additionally, oxygenation may aid in tumor growth reduction and anemia treatments by improving oxygen delivery (14).
Handajani et al. studied 108 diabetics and found that oxygenated water reduced postprandial glucose levels, particularly in participants with normal nutritional status, who also exhibited a significant decrease in malondialdehyde (MDA) levels after 45 days of consumption. Many participants reported feeling healthier overall (15). Fang et al. discovered that high doses of oxygenated water normalized serum uric acid levels in hyperuricemic rats, suggesting its potential to enhance uric acid metabolism compared to normal water (16).
Conversely, some studies have shown no effectiveness of oxygenated water (6, 17). It was hypothesized that additional oxygen in the water might offset oxygen loss and improve athletic performance. However, Piantadosi refuted this, citing a significant decrease in oxygen content upon opening the bottle and noting that the intestine is not designed for gas exchange, resulting in negligible oxygen absorption (17). King et al. reported that a drink with nano-bubble oxygen improved cycling performance, increasing time-trial speed by 2.4% and peak power during the Wingate test by 7.1%, suggesting potential benefits for competitive cyclists (18). However, Tiller and Jeukendrup challenged these findings, citing conflicting physiological evidence (19).
A systematic review of eight studies found limited evidence supporting health benefits of alkaline, oxygenated, or demineralized water compared to mineral water. Alkaline water showed no health advantage over mineral water. One study suggested that consuming oxygenated water (190 mL of dissolved oxygen per gram) for at least 14 days increased ascorbyl radicals in the body. In two studies, no significant differences were observed between the oxygenated and mineral water groups post-exercise. Regular consumption of demineralized water showed inferior results compared to mineral water (5).
In the study by Leibetseder et al., twenty healthy males were randomized into two groups: Group A (O2-water) and group B (control water). The intervention group consumed water containing 180 mg O2/L before exercise, which resulted in a significant difference in blood lactate concentration post-exercise. However, no differences were observed in parameters such as achievable power, heart rate, oxygen absorption, and respiration rate between the two groups. The observed difference in blood lactate concentration may have been influenced by the statistical test used rather than the intervention itself (20). Similarly, in the Wing-Gaia study, which compared water containing 110 mg O2/L to normal mineral water, no significant improvement was found in pre- and post-exercise performance (21). Overall, supplemental oxygen enhances performance during exercise but not prior to or between bouts (17).
To achieve optimal results with oxygen-rich water, several key considerations must be addressed. First, the oxygen concentration in the water should meet standard levels, as lower concentrations can reduce its efficacy. Containers must be securely sealed to prevent oxygen leakage. It is advisable to store the container in the refrigerator and shake it before use. Additionally, since oxygen levels drop significantly after opening, the water should be consumed immediately. Unlike Pakdaman's study, which indicated that oxygen consumption continued to elevate blood oxygen levels for 3 - 4 hours (2), this study suggests that higher doses of oxygen-rich water consumed at shorter intervals may yield better results, depending on the patient’s blood oxygen saturation.
5.1. Conclusions
The findings suggest that, under suitable conditions, oxygen-rich water (modified for mineral composition, oxygen content, and pH) can serve as a symptomatic treatment to enhance the clinical condition of hospitalized patients with hypoxia, including those with SARS-CoV-2.