1. Background
Urinary tract infections (UTIs) affect over 150 million individuals worldwide annually, making them one of the most prevalent illnesses globally and a significant challenge for healthcare systems (1). Healthcare-associated infections (HAIs) represent a major global health concern, arising from various factors such as substandard hospital conditions and the use of invasive medical equipment like catheters and probes. Among these, catheter-related infections (CRIs) are particularly common and are associated with increased healthcare costs, prolonged hospital stays, and heightened morbidity and mortality (2, 3). Approximately 15 - 25% of hospitalized patients require urinary catheters (UCs). These devices are utilized in patients with surgical needs or mobility issues to manage urine drainage, urinary retention, or urinary incontinence, and are used for both male and female patients (4, 5).
According to the centers for disease control and prevention (CDC), catheter-associated urinary tract infections account for approximately 13,000 deaths annually in the United States and contribute to 75% of hospital-acquired infections (6). Studies indicate that 32.2% of HAIs in intensive care units (ICUs) are UTIs, with nearly 80% linked to urinary catheters. This high prevalence is primarily due to the extensive use of catheters in critically ill patients (7-9). A study conducted in Kermanshah reported an incidence of hospital-acquired urinary tract infections (HAUTIs) of approximately 4.5% among ICU patients, with notable variations across hospital wards (10).
Indwelling urinary catheters are the leading cause of catheter-associated UTIs (CAUTIs). The risk of infection increases by 3 - 7% for each day a patient remains catheterized. According to the National Healthcare Safety Network (NHSN) and the CDC, a UTI is defined as an infection caused by a urinary catheter left in place for more than two consecutive days. To meet this definition, the patient must also exhibit at least one of the following symptoms: Fever (≥ 38.0°C), suprapubic tenderness, or costovertebral angle pain or soreness (6, 11). The majority of bacteria isolated in CAUTIs are gram-negative bacteria (GNB), including Escherichia coli, Klebsiella species (e.g., K. aerogenes), Proteus mirabilis, and Pseudomonas aeruginosa. Gram-positive bacteria (GPB), such as Staphylococcus aureus and Enterococcus species, are also significant pathogens (12-14).
For most CAUTI infections, the patient's own gut microbiota serves as the reservoir for pathogens like E. coli and Klebsiella spp., while multi-drug-resistant (MDR) strains are often acquired from external sources (15, 16). These superbugs are occasionally transmitted as nosocomial infections, particularly in ICU settings (17). Common antibiotics are typically used to treat UTIs; however, if these infections are not identified and treated promptly, serious complications may arise. These include high blood pressure, kidney failure, uremia, premature birth, and miscarriage in pregnant women. The improper use of antimicrobial medications in treating UTIs has contributed to the spread of antibiotic resistance and the emergence of MDR uropathogens.
Given the shift in the causes of UTIs and the increasing resistance of uropathogens to antibiotics, it is crucial to determine the causative pathogens and conduct drug sensitivity testing. These steps are essential for the effective treatment of UTI patients and have been shown to reduce mortality rates and overall hospital expenses (18). In most cases, treatment is initiated empirically, as sensitivity and resistance reports typically require at least two days. Therefore, understanding the antibiotic resistance patterns of uropathogenic bacteria is highly beneficial for guiding empirical treatment. Importantly, the pattern of antibiotic resistance varies over time and across regions, making ongoing research on these patterns critical for the effective management of UTI-related infections (19).
2. Objectives
The present study aimed to investigate the frequency of bacterial etiological factors and the antibiotic resistance of infections associated with urinary catheters in patients hospitalized in Khorramabad teaching hospitals during 2022 - 2023.
3. Methods
3.1. Study Design and Data Collection
This retrospective study was conducted on patient records of individuals with urinary catheters admitted to Khorramabad teaching hospitals in 2022 - 2023. The researchers used the census method to examine the patients according to the following criteria: Inclusion criteria: Patients with urinary catheters admitted to Khorramabad teaching hospitals in 2022 who had urine cultures available. Exclusion criteria: (1) Patients with a history of urinary infections before catheter insertion; (2) Immunocompromised patients; (3) individuals under 18 years of age; (4) clinical records missing urine culture results; (4) outpatients, (5) repeat clients or patients. After applying these criteria, 250 individuals were included in the study.
The researchers collected data using a researcher-designed checklist that included information extracted from patient medical records, such as: (1) Demographics: Age and sex; (2) medical history: Previous diseases and conditions; (3) hospitalization details: Number of days hospitalized and the department of admission; (4) microbiological findings: Types of bacteria reported from urine cultures and antibiotic resistance results; (5) clinical details: Antibiotic regimens derived from bacterial culture results and the presence or absence of urinary symptoms.
3.2. Isolation
Between March 2022 and March 2023, records of 250 patients with urinary catheters hospitalized in teaching hospitals in Khorramabad were reviewed. These patients had urine cultures available for analysis. Midstream urine samples were collected using sterile plastic containers to ensure contamination-free sampling. The collected samples were promptly processed using various laboratory methods, including Gram staining, microbial culture, and antibiotic susceptibility testing.
3.3. Identification of Bacteria
The identification of catheter-associated urinary tract infections (CAUTIs) involved three main laboratory steps:
3.3.1. Sample Collection
A sterile urine sample was collected directly from the catheter to prevent contamination and ensure the accuracy of microbial analysis.
3.3.2. Microbial Culture
The collected samples were cultured on MacConkey agar and blood agar. These media allowed for the identification of bacterial species and differentiation between gram-susceptible and gram-resistant bacteria.
3.3.3. Microscopic Analysis
The urine samples were examined under a microscope to detect the presence of bacteria and white blood cells, confirming infection and providing additional diagnostic information.
3.4. Antibiotic Susceptibility
The antibiotic susceptibility of the isolates was assessed using the Kirby-Bauer disk diffusion method. For this procedure, Müller-Hinton agar plates were prepared, inoculated with the bacterial isolates, and incubated at 37°C for 24 hours. The zones of bacterial inhibition around the antibiotic disks were measured according to the clinical and laboratory standards institute (CLSI) 2013 guidelines. Intermediate susceptibility was classified as resistant for analytical purposes.
Sensitivity testing was conducted on both gram-negative and gram-positive bacterial isolates using 11 antibiotics commonly prescribed for catheter-associated UTIs. These antibiotics included: Ampicillin (30 µg), amoxicillin (25 µg), cefotaxime (30 µg), ceftazidime (30 µg), ceftriaxone (30 µg), imipenem (10 µg), amikacin (30 µg), gentamycin (30 µg), ciprofloxacin (10 µg), nalidixic acid (30 µg), kanamycin (30 µg).
3.5. Statistical Analysis
Data were analyzed using Stata version 16 statistical software. Descriptive statistics, including mean and standard deviation, were calculated for quantitative variables, while frequency and percentage were reported for qualitative variables. Chi-square tests and independent t-tests were used to assess associations between variables. Additionally, multivariable logistic regression analysis was performed to examine the relationships between different variables and the prevalence of infections and antibiotic resistance. A significance level of 0.05 was considered for all statistical analyses.
4. Results
4.1. Demographic and Clinical Information of Patients
The average age of the patients in this study was 53.60 ± 10.86 years, with 51.87 ± 12.01 years for men and 54.52 ± 10.12 years for women. The results also indicated that the average duration of hospitalization was 6.32 ± 1.98 days, with 6.20 ± 2.0 days for men and 6.38 ± 1.97 days for women. In terms of gender distribution, 65.20% of the study population were female. Regarding marital status, 81.20% of the patients were married, while 18.80% were single. Clinical records revealed that 18.40% (46 patients) had a positive history of underlying diseases, and 96.40% (241 patients) had a positive history of antibiotic use. The highest proportion of patients, 24.80% (62 patients), were hospitalized in the internal medicine department. Further details on the frequency distribution of clinical characteristics and inpatient department information are summarized in Table 1.
| Variables and Category | Frequency (%) |
|---|---|
| Gender | |
| Male | 87 (34.80) |
| Female | 163 (65.20) |
| Married status | |
| Married | 203 (81.20) |
| Unmarried | 47 (18.80) |
| History of underlying disease | |
| Yes | 46 (18.40) |
| No | 204 (81.60) |
| Previous history of antibiotic use | |
| Yes | 241 (96.40) |
| No | 9 (3.60) |
| Inpatient department | |
| Emergency | 49 (19.60) |
| Internal | 62 (24.80) |
| Surgery | 39 (15.60) |
| Women | 24 (9.60) |
| Poisoning | 1 (0.40) |
| General | 21 (8.40) |
| Infectious | 54 (21.60) |
| Urinary symptoms | |
| Yes | 216 (86.40) |
| No | 34 (13.60) |
4.2. Identification Bacteria
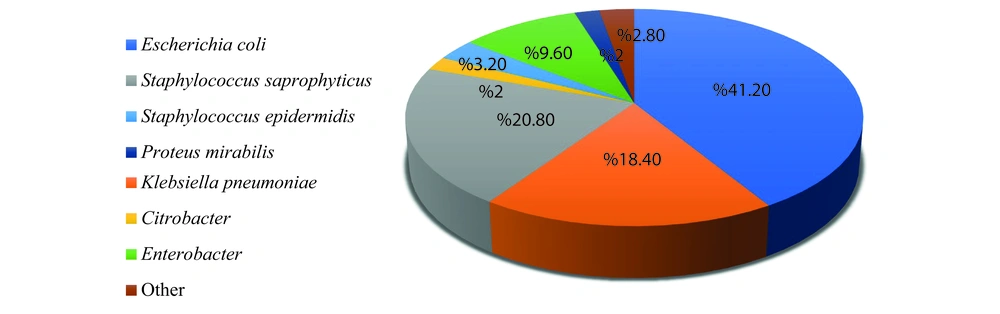
The results revealed that the most frequently reported pathogen was E. coli (41.20%), followed by Staphylococcus saprophyticus (20.80%) and Klebsiella pneumoniae (18.40%). The pathogens with the lowest frequency were Citrobacter and P. mirabilis, each accounting for 2% of cases (Figure 1).
4.3. Antibiotic Resistance Rate
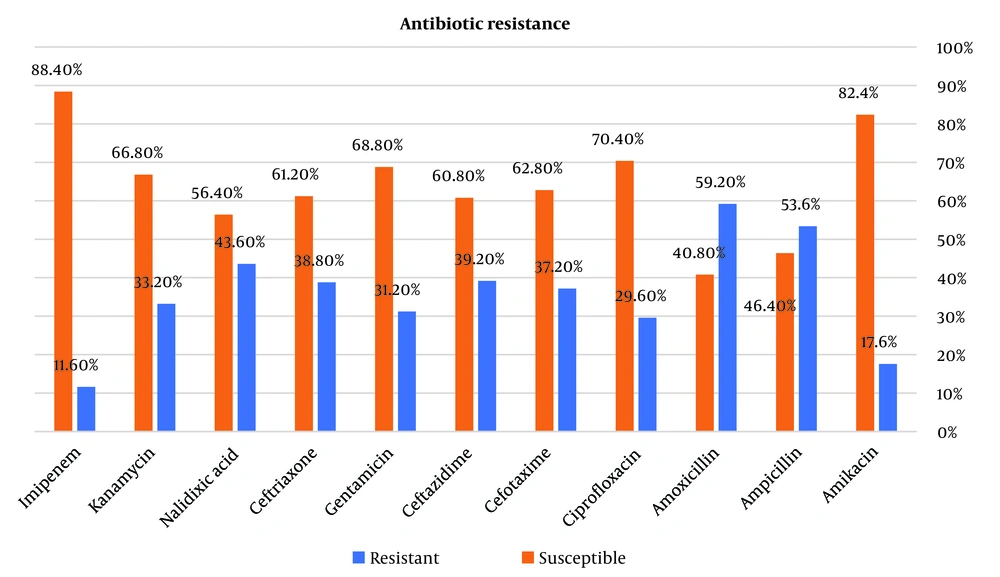
The results of the study showed that the highest antibiotic resistance was reported to amoxicillin and ampicillin in the studied patients (59.2% and 53.6%, respectively). Also, the lowest antibiotic resistance was reported in the studied patients, respectively, compared to imipenem (11.6%) and then amikacin (17.6%). Other information is given in Figure 2. A chi-square test has been used to check the status of antibiotic resistance according to age and gender. The results of Table 2 showed that in people less than 50 years old, the highest resistance was shown by amoxicillin with 71.11% (64 patients) and ampicillin with 68.89% (62 patients). Also, in these people, the lowest antibiotic resistance was observed in Imipenem with 16.67% (15 patients) and amikacin with 24.44% (22 patients). Also, the results showed that people over 50 years old, amoxicillin (52.50%) and ampicillin (45%) showed the highest resistance. Also, in these people, the lowest antibiotic resistance was observed with imipenem (8.75%) and amikacin with 13.75%. The chi-square test showed that there was no statistically significant relationship between the antibiotic resistance of the studied antibiotics and the age of the patients (P > 0.05). Other information is given in Table 2. Based on the results of Table 2, the highest resistance to amoxicillin and ampicillin was observed in women and men, respectively. Also, these people showed the least resistance to Imipenem and amikacin. According to P > 0.05, there was no statistically significant relationship between antibiotic resistance, the ratio of antibiotic types, and the gender of patients.
| Antibiotic Resistance | Age (y) | P-Value b | Gender | P-Value b | ||
|---|---|---|---|---|---|---|
| < 50 | > 50 | Male | Female | |||
| Amikacin | 0.053 | 0.133 | ||||
| Resistant | 22 (24.44) | 22 (13.75) | 11 (12.64) | 33 (20.25) | ||
| Susceptible | 68 (75.56) | 138 (86.25) | 76 (87.36) | 130 (79.75) | ||
| Ampicillin | 0.098 | 0.866 | ||||
| Resistant | 62 (68.89) | 72 (45) | 46 (52.87) | 88 (53.99) | ||
| Susceptible | 28 (31.11) | 88 (55) | 41 (47.13) | 75 (46.01) | ||
| Amoxicillin | 0.056 | 0.686 | ||||
| Resistant | 64 (71.11) | 84 (52.50) | 53 (60.92) | 95 (58.28) | ||
| Susceptible | 26 (28.89) | 76 (47.50) | 34 (39.08) | 68 (41.72) | ||
| Ciprofloxacin | 0.066 | 0.217 | ||||
| Resistant | 33 (36.67) | 41 (25.63) | 30 (34.48) | 44 (26.99) | ||
| Susceptible | 57 (63.33) | 119 (74.38) | 57 (65.52) | 119 (73.01) | ||
| Cefotaxime | 0.132 | 0.861 | ||||
| Resistant | 39 (43.33) | 54 (33.74) | 33 (37.93) | 60 (36.81) | ||
| Susceptible | 51 (56.67) | 106 (66.25) | 54 (62.07) | 103 (63.19) | ||
| Ceftazidime | 0.070 | 0.606 | ||||
| Resistant | 42 (46.67) | 56 (35) | 36 (41.38) | 62 (38.04) | ||
| Susceptible | 48 (53.33) | 104 (65) | 51 (58.62) | 101 (61.96) | ||
| Gentamicin | 0.585 | 0.595 | ||||
| Resistant | 30 (33.33) | 48 (30) | 29 (33.33) | 49 (30.06) | ||
| Susceptible | 60 (66.67) | 112 (70) | 58 (66.67) | 114 (69.94) | ||
| Ceftriaxone | 0.056 | 0.735 | ||||
| Resistant | 42 (46.67) | 55 (34.38) | 35 (40.23) | 62 (38.04) | ||
| Susceptible | 48 (53.33) | 105 (65.63) | 52 (59.77) | 101 (61.96) | ||
| Nalidixic Acid | 0.640 | 0.175 | ||||
| Resistant | 41 (45.56) | 68 (42.50) | 43 (49.43) | 66 (40.49) | ||
| Susceptible | 49 (54.44) | 92 (57.50) | 44 (50.57) | 97 (59.51) | ||
| Kanamycin | 0.383 | 0.595 | ||||
| Resistant | 33 (36.67) | 50 (31.25) | 27 (31.03) | 56 (34.36) | ||
| Susceptible | 57 (63.33) | 110 (68.75) | 60 (68.97) | 107 (65.64) | ||
| Imipenem | 0.061 | 0.970 | ||||
| Resistant | 15 (16.67) | 14 (8.75) | 10 (11.49) | 19 (11.66) | ||
| Susceptible | 75 (83.33) | 146 (91.25) | 77 (88.51) | 144 (88.34) | ||
a Values are presented as No. (%).
b Chi-square test.
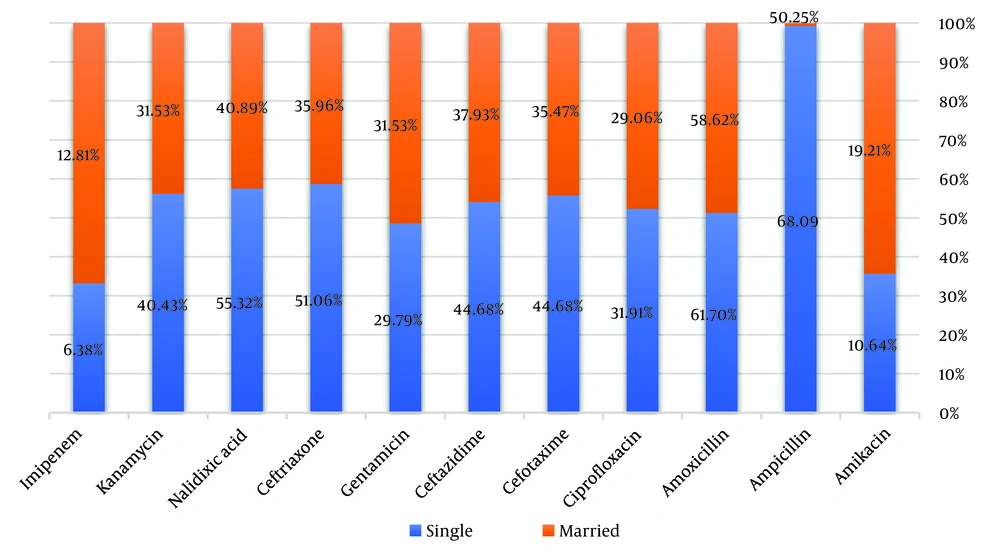
The results of Figure 3 show that married people showed the highest resistance to amoxicillin and ampicillin with 58.62% and 50.25%, respectively. Also, in single people, ampicillin (68.09%) and amoxicillin (61.70%) showed the highest antibiotic resistance. Based on the chi-square test, no significant relationship was found between marital status and antibiotic resistance (P > 0.05). A chi-square test was used to investigate the relationship between antibiotic resistance and antibiotic consumption. The results of the test showed that 59.75% of people who had a history of antibiotic use showed resistance to amoxicillin and 53.53% to ampicillin.
There was a statistically significant relationship between antibiotic resistance to ampicillin and amoxicillin and a previous history of antibiotic use in the patients of the study population (P < 0.05). Also, the lowest antibiotic resistance with a previous history of antibiotic use was observed in imipenem with 11.20% and amikacin with 17.43%, and there was a statistically significant relationship between antibiotic resistance to imipenem and previous history of antibiotic use (P < 0.05). Other details related to antibiotic resistance in the studied population with a previous history of antibiotic use can be seen in Table 3. According to the results of Table 3, in people with urinary symptoms, 55.56% (120 people) had resistance to ampicillin, and 59.72% (129 people) had resistance to amoxicillin. Also, 12.50% (27 people) had resistance to imipenem. Based on the chi-square test, there was no statistically significant association between antibiotic resistance and urinary symptoms (P > 0.05).
| Antibiotic Resistance | Antibiotic Use | P-Value b | Urinary Symptoms | P-Value b | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Amikacin | 0.711 | 0.148 | ||||
| Resistant | 42 (17.43) | 199 (82.57) | 41 (18.98) | 175 (81.02) | ||
| Susceptible | 2 (22.22) | 7 (77.78) | 3 (8.82) | 31 (91.18) | ||
| Ampicillin | 0.048 | 0.118 | ||||
| Resistant | 129 (53.53) | 112 (46.47) | 120 (55.56) | 96 (44.44) | ||
| Susceptible | 5 (55.56) | 4 (44.44) | 14 (41.18) | 20 (58.82) | ||
| Amoxicillin | 0.009 | 0.672 | ||||
| Resistant | 144 (59.75) | 97 (40.25) | 129 (59.72) | 87 (40.28) | ||
| Susceptible | 4 (44.44) | 5 (55.56) | 19 (55.88) | 15 (44.12) | ||
| Ciprofloxacin | 0.803 | 0.058 | ||||
| Resistant | 71 (29.46) | 170 (70.54) | 69 (31.94) | 147 (68.06) | ||
| Susceptible | 3 (33.33) | 6 (66.67) | 5 (14.71) | 29 (85.29) | ||
| Cefotaxime | 0.807 | 0.312 | ||||
| Resistant | 90 (37.34) | 151 (62.66) | 83 (38.43) | 133 (61.57) | ||
| Susceptible | 3 (33.33) | 6 (66.67) | 10 (29.41) | 24 (70.59) | ||
| Ceftazidime | 0.713 | 0.102 | ||||
| Resistant | 95 (39.42) | 146 (60.58) | 89 (41.20) | 127 (58.80) | ||
| Susceptible | 3 (33.33) | 6 (66.67) | 9 (26.47) | 25 (73.53) | ||
| Gentamicin | 0.382 | 0.151 | ||||
| Resistant | 74 (30.71) | 167 (69.29) | 71 (32.87) | 145 (67.13) | ||
| Susceptible | 4 (44.44) | 5 (55.56) | 7 (20.59) | 27 (79.41) | ||
| Ceftriaxone | 0.732 | 0.407 | ||||
| Resistant | 94 (39) | 147 (61) | 86 (39.81) | 130 (60.19) | ||
| Susceptible | 3 (33.33) | 6 (66.67) | 11 (32.35) | 23 (67.65) | ||
| Nalidixic Acid | 0.959 | 0.155 | ||||
| Resistant | 105 (43.57)) | 136 (56.43) | 98 (45.37) | 118 (54.63) | ||
| Susceptible | 4 (44.44) | 5 (55.56) | 11 (32.35) | 23 (67.65) | ||
| Kanamycin | 0.993 | 0.198 | ||||
| Resistant | 80 (33.20) | 161 (66.80) | 75 (34.72) | 141 (65.28) | ||
| Susceptible | 3 (33.33) | 6 (66.67) | 8 (23.53) | 26 (76.47) | ||
| Imipenem | 0.006 | 0.263 | ||||
| Resistant | 27 (11.20) | 214 (88.80) | 27 (12.50) | 189 (87.50) | ||
| Susceptible | 2 (22.22) | 7 (77.78) | 2 (5.88) | 32 (94.12) | ||
a Values are presented as No. (%).
b Chi-square test.
5. Discussion
Considering that many studies have not been conducted to evaluate the results of antibiotic resistance treatment in pathogens in the western region of Iran, the present study was conducted with the aim of investigating the frequency of bacterial etiological factors and antibiotic resistance of infections caused by urinary catheters in patients hospitalized in Khorramabad teaching hospitals in 2022 - 2023. Gender is one of the factors that influence urinary tract infections, according to several studies (20). Similar to the findings of the Mohammadi et al. study (21), women were more likely than men to have urinary infections (65.20%). The majority of the bacteria in the study by Shirvani et al. (22) were also isolated from samples provided by women. The urethra's short length, its close proximity to the anus and vagina, and the warm, humid environment of the perineum may be contributing factors to the rise in infections among women (23, 24). In the present study, the most common urinary pathogens in people were E. coli (41.20%), S. saprophyticus (20.80%), and K. pneumoniae (18.40%).
In studies conducted in other parts of Iran and the world, this microorganism is known as the most common cause of urinary tract infection. The prevalence of this bacterium in the study of Mohammed et al. (25), which was conducted on 117 samples in Bahrain, was 49.06%. In the study of Mohammed et al. in 2022, which was conducted on 773 samples in Somalia, E. coli was the most common pathogen (26.3%) (1). A study by Aktaş and Denktaş was conducted in 2020 with the aim of investigating the results of urine culture and antibiotic sensitivity of E. coli isolates in Turkey. E. coli accounted for 48% of the strains in this study, with Enterococcus coming in at 9%, Staphylococcus coagulase-negative at 8.3%, and Klebsiella at 7.6% (26).
Escherichia coli accounted for 50.6% of the organisms in the Tavanaee Sani and Mehrafarid study conducted in Mashhad. The most frequently occurring organisms were Candida albicans, Pseudomonas aeruginosa, and K. pneumoniae. Enterococcus was the most prevalent Gram-positive organism (27). According to a study conducted in 2020 in Mashhad by Vakilzadeh et al., the most prevalent urinary pathogens in individuals with positive cultures were E. coli (60.9%), K. pneumoniae (13.9%), and Enterococcus (28). The findings of the aforementioned studies demonstrate that urinary pathogen prevalence varies over time and across geographical locations. However, one thing is certain: E. coli is the most frequent cause of UTIs in the general population. The prevalence of other pathogens varies amongst studies.
Regardless of the bacterial strains, amoxicillin (59.2%) and ampicillin (53.6%) showed the highest resistance to UTI agents in both sexes in this study, and the findings are consistent with those of Baghani Aval et al. studies in Sabzevar (19) and Abedi Samakoosh et al. in Quemshar (29). Osman (30) also carried out a study in 2019 in Erbil, Iraq, to look into antibiotic resistance in bacteria that were isolated from urine samples of patients who had urinary tract infections. In the above study, the highest resistance to ampicillin, aztreonam, cefazolin, clindamycin, and tetracycline antibiotics was observed, which was consistent with the findings of the present study in terms of ampicillin resistance (30).
The study's findings about other antibiotics did not agree with ours, and this discrepancy might have been brought about by using different resistance assessment instruments and looking at a different number of antibiotics. For instance, the current study did not look into resistance to clindamycin, tetracycline, cefazolin, or aztreonam. In this study, the lowest rate of resistance in men and women was related to imipenem (11.6%) and then amikacin (17.6%). The results of the present study were consistent with the results of the study by Mohammadi et al. in Sanandaj (21). Similarly, in the study conducted by Safdari and Ghazvini in Mashhad (31) and the study conducted by Abdollahi and Mehr Azma (32) in Imam Khomeini Hospital, Tehran, the least resistance to imipenem and amikacin was reported. In the Tavanaee Sani and Mehrafarid study in Mashhad, the highest level of sensitivity to the antibiotic amikacin was observed (27).
In the study of Aktaş and Denktaş in 2020, the most effective antibiotics for Harvey E. coli included imipenem, meropenem, ertapenem, and amikacin. In terms of sensitivity to imipenem and amikacin, these two studies were consistent. However, in the present study, sensitivity to ciprofloxacin and gentamicin was also high, which was not reported in the above study (26). Considering these similarities regarding the lowest antibiotic resistance compared to amikacin and imipenem, and considering the same drug prescription pattern in different parts of the country, these two drugs can be proposed as the first line of empirical treatment for urinary tract infection. However, among the limitations of using these two drugs, the following can be mentioned: Amikacin may cause decreased kidney function, tinnitus, vertigo, and hearing loss in cases of improper administration (33). Imipenem can also cause kidney dysfunction and convulsions (34). Therefore, during administration, the maximum and minimum concentrations of the drug should be carefully monitored.
In this study, over 95% of patients had recently used antibiotics, and the findings indicated a statistically significant association between prior antibiotic use and resistance to amoxicillin, ampicillin, and imipenem (P < 0.05). This issue may explain why the level of antibiotic resistance in various organisms against the studied antibiotics was high. It might be possible to prevent the increasing rate of drug resistance with better and more appropriate use of antibiotics. Numerous studies in different parts of the world have shown that the cause and resistance pattern of urinary infections have changed (35). Therefore, identifying the bacterial agents that cause urinary infections and using appropriate and effective antibiotics to eliminate them are practical approaches to dealing with these infections and preventing their consequences. A high prevalence of microbial resistance to common drugs increases treatment costs, as the lack of effectiveness of cheaper, commonly used drugs often necessitates switching to more expensive alternatives (36). This underscores the importance of making informed decisions regarding treatment.
The large variation in antibiotic resistance levels can be attributed to differences in the use of antibiotics for treating the disease in different regions. It is well-known that inappropriate and excessive use of antibiotics leads to bacterial resistance. Reducing the prescription of certain antibiotics can, in turn, reduce resistance. The high rate of bacterial resistance to antibiotics observed in the present study, as well as in other studies conducted in Iran compared to other countries, can likely be attributed to indiscriminate antibiotic prescriptions and self-administration without a doctor’s guidance. To address this issue, measures such as performing microscopic urine examinations and urine cultures, prescribing antibiotics properly based on culture and antibiogram results, and periodically conducting similar research in different times and locations to better understand the epidemiology of urinary infections and the resistance and sensitivity levels of microorganisms to various antibiotics should receive greater attention (37, 38).
The limitations of this study included deficiencies in the clinical and laboratory data of some patients. In certain cases, researchers were unable to access complete and accurate information for specific individuals, leading to their exclusion from the study.
5.1. Conclusions
The majority of bacterial isolates common in catheter-associated urinary tract infections suggest that amikacin and imipenem are appropriate options for initiating empirical therapy until antibiogram results are available. Given the importance of emerging antibiotic resistance and the changing susceptibility and resistance patterns of bacteria, performing antibiogram testing is essential for the effective treatment of these infections.