1. Background
Streptococcus pneumoniae infection is a common cause of community-acquired pulmonary infections in children and significantly increases the risk of death in pediatric populations (1, 2). Antibiotics remain the primary treatment for pulmonary infections in children; however, the irrational use of antibiotics and rising antibiotic resistance in clinical settings have impacted the effectiveness of these treatments. OM85-BV, a bacterial lysate, is an oral immunomodulator that helps regulate immune function. When administered, OM85-BV stimulates the synthesis of globulin antibody molecules with antibody activity or structural components, enhancing the patient's resistance to infections and helping delay disease progression (3-5).
Bene et al. (6) found that oral administration of OM85-BV increased the number of specific antibody cells targeting S. pneumoniae, S. pyogenes, Haemophilus influenzae, and Klebsiella pneumoniae in the tonsil tissues of children undergoing tonsillectomy. This finding suggests that OM85-BV could be useful in the clinical management of pulmonary infections, including those caused by S. pneumoniae.
In children, immune function is still underdeveloped, and the respiratory system's defense mechanisms are weak. As a result, bacterial infections of the respiratory tract often spread to the lungs, causing serious infections. T lymphocyte subsets and immunoglobulins are essential components for regulating the body’s immune response (7). Furthermore, the toll-like receptor (TLR)/nuclear factor kappa-B (NF-κB) pathway is a critical regulatory mechanism of the inflammatory response. Toll-like receptors, which are a class of recognition receptors for type I transmembrane glycoproteins, are widely expressed in alveolar macrophages, bronchial epithelial cells, and cancer cells (8). When pathogens invade, TLRs activate the immune system and participate in the pathophysiological processes of both acute and chronic airway inflammation (9).
Toll-like receptors, a cell surface receptor, plays a significant role when bound to lipopolysaccharides (LPS) from bacterial cell walls, activating the NF-κB transcription factor pathway. This process promotes the production and release of pro-inflammatory cytokines (10). Domon et al. (11) demonstrated that the expression of TLR-related pro-inflammatory factors increased in the bronchoalveolar lavage fluid (BALF) of a mouse model induced by S. pneumoniae, indicating that the TLR/NF-κB pathway is involved in the regulation of S. pneumoniae pulmonary infections. Despite these findings, there is relatively little clinical research on the effect of bacterial lysates OM85-BV on S. pneumoniae pulmonary infections in children and its impact on TLR/NF-κB pathway signaling. This underscores the need for further investigation to understand the full potential of OM85-BV in pediatric pulmonary infections and its role in immune response modulation.
2. Objectives
Therefore, this study included children with S. pneumoniae pulmonary infection to explore the mechanism of the TLRs/NF-κB pathway in S. pneumoniae pulmonary infections using bacterial lysates OM85-BV. The goal was to provide valuable insights and reference points for the clinical diagnosis and treatment of S. pneumoniae pulmonary infections in pediatric patients.
3. Methods
3.1. Clinical Data
From March 2022 to June 2023, children with lower respiratory tract infections, accompanied by varying degrees of high fever, chills, and other clinical symptoms, were admitted to the Department of Pediatrics at Tongde Hospital of Zhejiang Province. Among these, 120 children were diagnosed with S. pneumoniae pulmonary infection through sputum isolation, culture, chest X-ray, and other examinations.
Inclusion criteria: (1) Children who met the diagnostic criteria for S. pneumoniae pulmonary infection; (2) age less than 10 years; (3) onset within 0 - 3 days; (4) family members were informed about the study, voluntarily participated, and provided signed informed consent. Exclusion criteria: (1) Other pulmonary infectious diseases; (2) nasal congenital dysplasia; (3) significant organ dysfunction; (4) allergic to therapeutic drugs; (5) recent treatment history with immunomodulators. The experiment was approved by the Tongde Hospital of Zhejiang Province Ethics Committee.
A total of 120 patients were randomly divided into two groups: (1) The control group; and (2) the OM85-BV group, with 60 cases in each. In the control group, there were 35 males and 25 females, with a mean age of 4.1 ± 1.5 years (range: 0.8 - 8.5 years). The average body temperature was 38.3 ± 1.0°C (range: 37.9 - 40.1°C), and body weight ranged from 12 to 30 kg (mean: 18.0 ± 4.3 kg). Imaging features in the control group suggested lung texture thickening, inflammatory infiltration, and parenchymal lesions in 38 cases, an air bronchogram in 34 cases, and a small amount of pleural effusion in 16 cases. In the OM85-BV group, there were 36 males and 24 females, with a mean age of 4.2 ± 1.8 years (range: 0.5 - 8.1 years). The average body temperature was 38.5 ± 1.1°C (range: 37.8 - 39.7°C), and body weight ranged from 12 to 30 kg (mean: 18.3 ± 3.6 kg). Imaging features in this group suggested lung texture thickening, inflammatory infiltration, and parenchymal lesions in 35 cases, an air bronchogram in 32 cases, and a small amount of pleural effusion in 15 cases. There were no significant differences between the two groups in terms of gender, age, weight, body temperature, or the proportion of imaging features (P > 0.05).
3.2. Treatment Plan
All patients received basic treatment, including oxygen inhalation, fever reduction, phlegm resolution, and cough relief. The control group was treated with intravenous mezlocillin (H37020221, Xinhua Pharmaceutical (Gaomi) Co., LTD., China) at 100 mL/kg/day, administered once daily for 10 days. In addition to this treatment, the OM85-BV group received oral OM85-BV capsules (SJ20150041, OM Pharma SA, Switzerland), 3.5 mg per capsule, taken on an empty stomach every morning, once daily for 10 days.
3.3. Observation Indexes and Efficacy Evaluation
(1) Basic indicators: The time for clinical symptom disappearance and recovery of chest X-ray findings were recorded.
(2) Pulmonary function indicators: After treatment, changes in pulmonary function, including respiratory rate, tidal volume, volume to peak expiratory flow as a proportion of exhaled volume, and time to peak tidal expiratory flow as a proportion of expiratory time, were evaluated.
(3) Inflammatory markers: Blood samples were collected before (day 0) and after (day 10) treatment. In the morning, 3 mL of venous blood was drawn from each patient in a fasting state, and serum was collected using anticoagulant separation (Becton, Dickinson and Company, USA). Serum levels of C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and soluble interleukin-2 receptor (sIL-2R) were measured using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Jiwei Biotechnology Co., Ltd, China).
(4) Immune function indicators: The proportions of T lymphocyte subsets CD3+, CD4+, and CD8+ in serum were determined using a CytoFLEX S flow cytometer (Beckman Coulter, USA). The CD4+/CD8+ ratio was computed. The concentrations of IgA, IgG, and IgM in serum were also measured using immunoglobulin ELISA kits (Wuhan Elabscience Biotechnology Co., Ltd, China).
(5) Toll-like receptors/NF-κB pathway indicators: Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation (Ficoll® 400, Merck KGaA, Darmstadt, Germany). Total protein was extracted from the lysed cells and quantified. After gel electrophoresis and protein separation, the proteins were transferred to polyvinylidene fluoride membranes for sealing. Rabbit polyclonal TLR4 (1:1000), NF-κB (1:2000), p-NF-κB (1:1000) (Thermo Fisher Scientific Inc., USA), and mouse monoclonal β-actin (1:5000) primary antibodies were incubated overnight at 4°C. Horseradish peroxidase (HRP) IgG secondary antibody (1:2000) was applied, incubated at 25°C for 2 hours. The relative gray values of TLR4 and related protein expressions were detected using a gel imaging technique.
(6) The therapeutic effect in children was classified into three categories: Markedly effective, where clinical symptoms completely disappeared and the chest X-ray showed no shadow in the lung; effective, where clinical symptoms improved significantly and the chest X-ray indicated a clear reduction in the lung shadow; and ineffective, where there was no change or an aggravation of clinical symptoms, and the chest X-ray showed no reduction or an increase in the lung shadow. The total effective rate was calculated as follows:
(7) Adverse reactions: Any adverse reactions such as nausea/vomiting, diarrhea/abdominal pain, rash, fatigue, and cough were recorded during the treatment period.
3.4. Statistical Analysis
SPSS 22.0 statistical software was used for the analysis. Continuous data following a normal distribution were expressed as mean ± standard deviation (SD), and Student's t-test was used. Qualitative data were presented as frequency (%) and analyzed using the χ2 test. A P-value < 0.05 was considered statistically significant.
4. Results
4.1. Improvement of Clinical Symptoms of Subjects
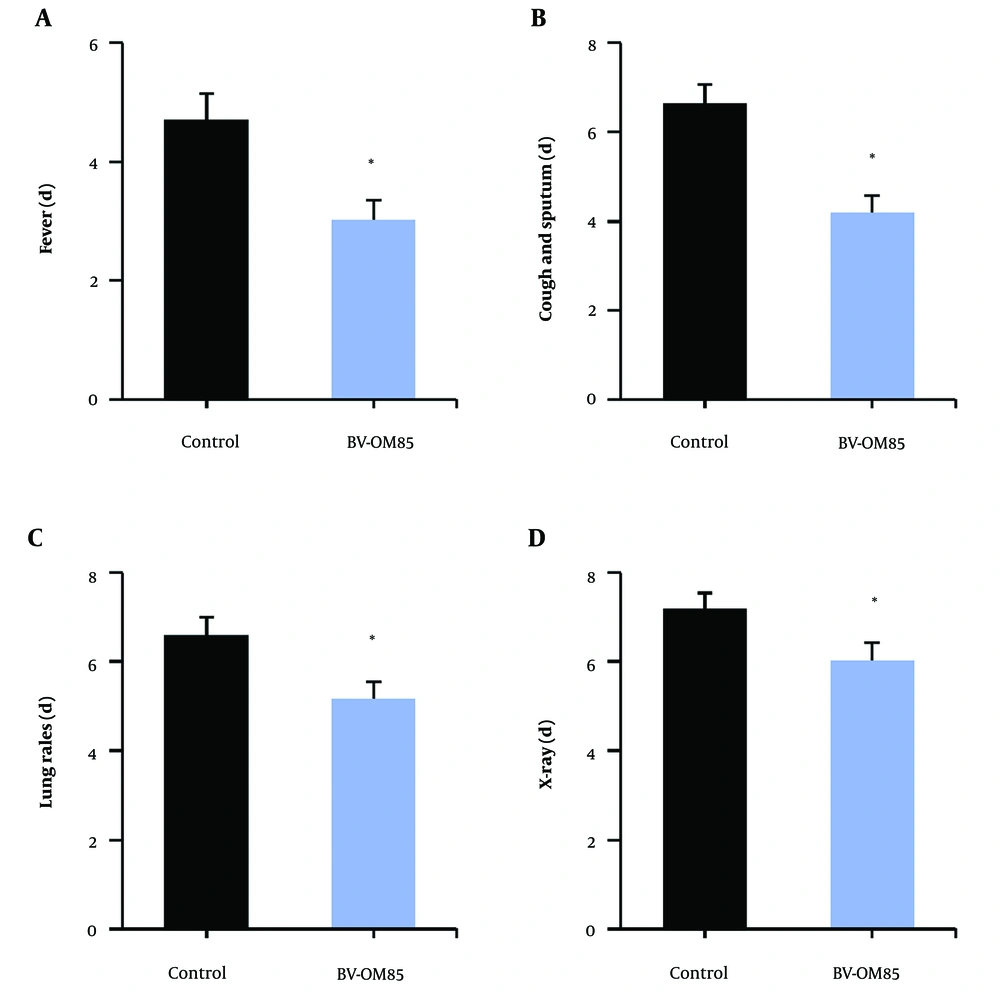
Figure 1 illustrates that, compared to the control group, the OM85-BV group had a significantly shorter time to improvement in clinical symptoms, including fever, cough, expectoration, pathological breath sounds in the lungs, and recovery on chest X-ray (P < 0.05).
Contrast of time to improvement of clinical symptoms in children. A, is the time of fever subsidence; B, is the disappearance time of cough and phlegm; C, is the disappearance time of lung pathological breath sounds; D, is the improvement time of chest X-ray symptoms; * as against the control group, P < 0.05.
4.2. Improvement of Lung Function of Subjects
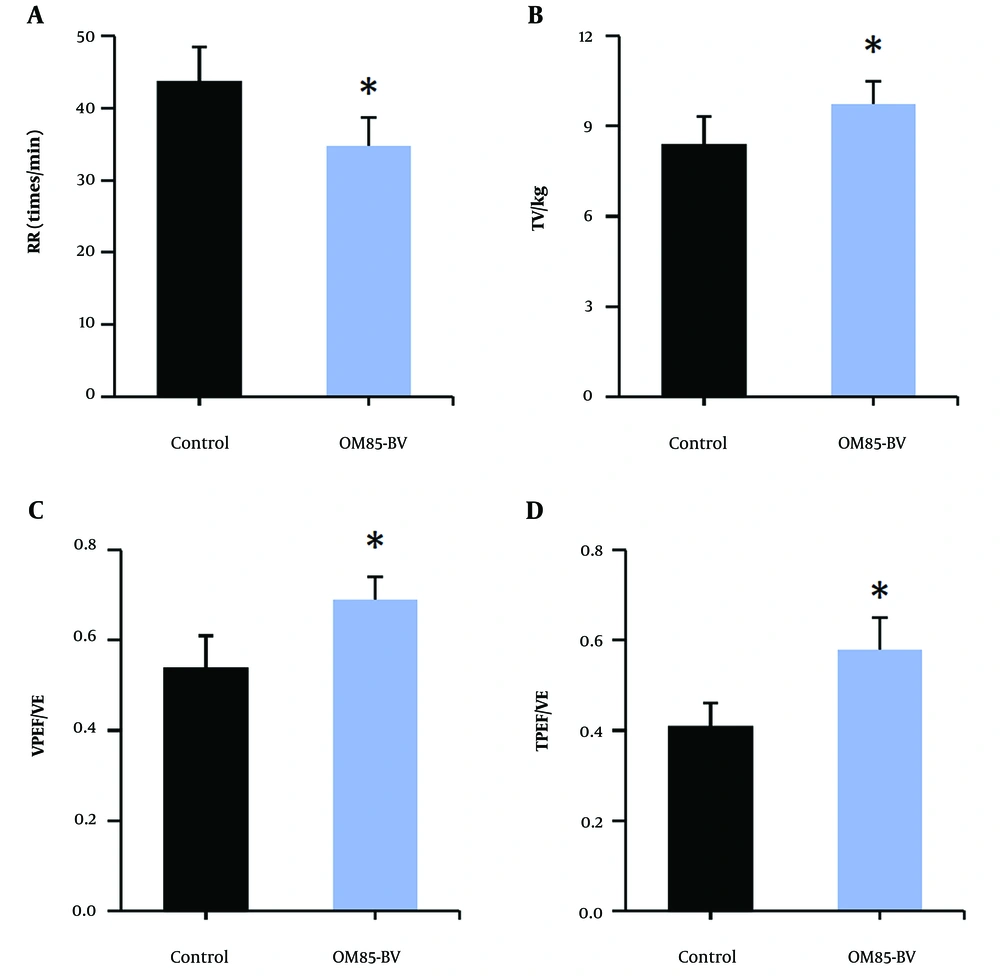
Figure 2 illustrates the subjects’ respiratory rate, tidal volume/kg, volume to peak expiratory flow as a proportion of exhaled volume, and time to peak tidal expiratory flow as a proportion of expiratory time, along with other lung function indexes. Compared to the control group, the OM85-BV group showed a significant decrease in respiratory rate, while tidal volume/kg, volume to peak expiratory flow as a proportion of exhaled volume, and time to peak tidal expiratory flow as a proportion of expiratory time were significantly increased (P < 0.05).
4.3. Improvement of Inflammatory Response in Subjects
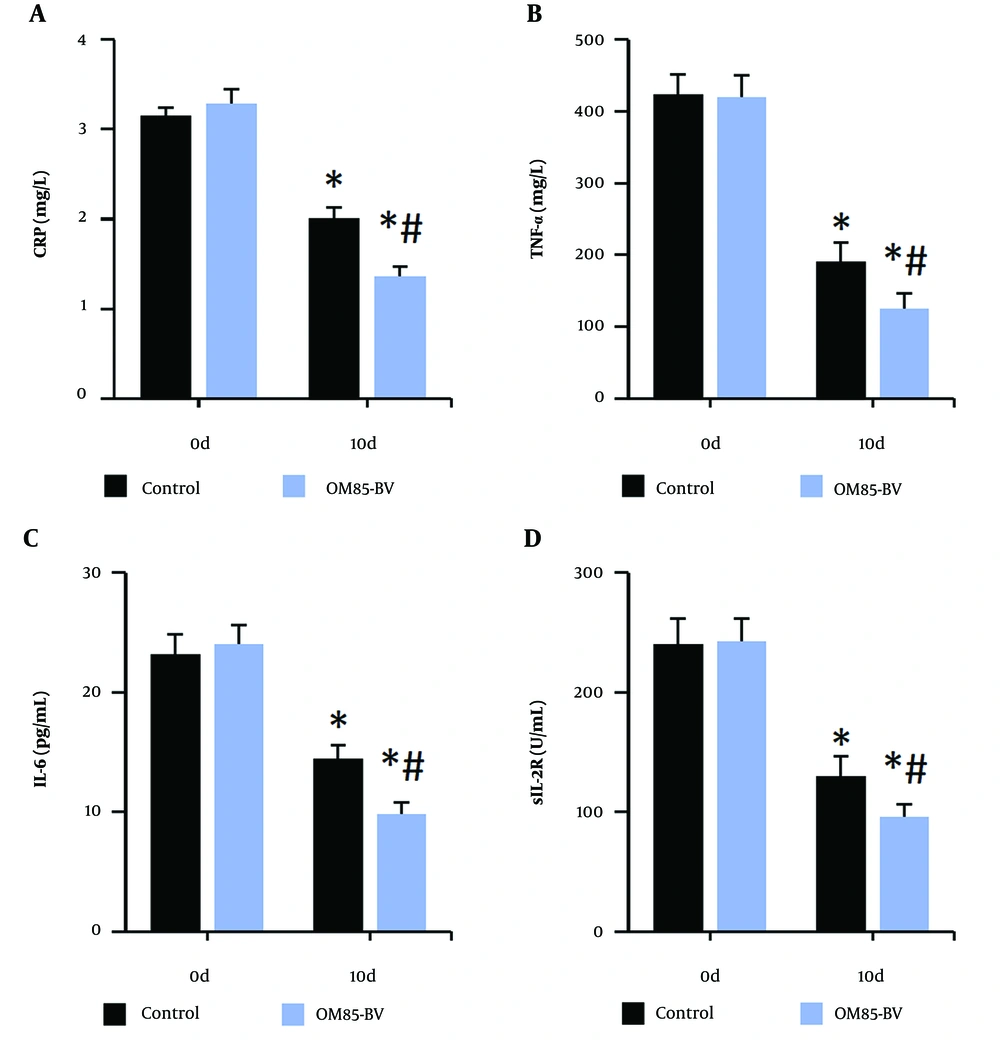
Figure 3 illustrates that the levels of CRP, TNF-α, IL-6, and sIL-2R in the peripheral blood of subjects were significantly lower after treatment compared to pre-treatment levels. Furthermore, after treatment, the levels of these inflammatory markers in the OM85-BV group were significantly lower compared to the control group (all P < 0.05).
4.4. Improvement of Immune Function of Subjects
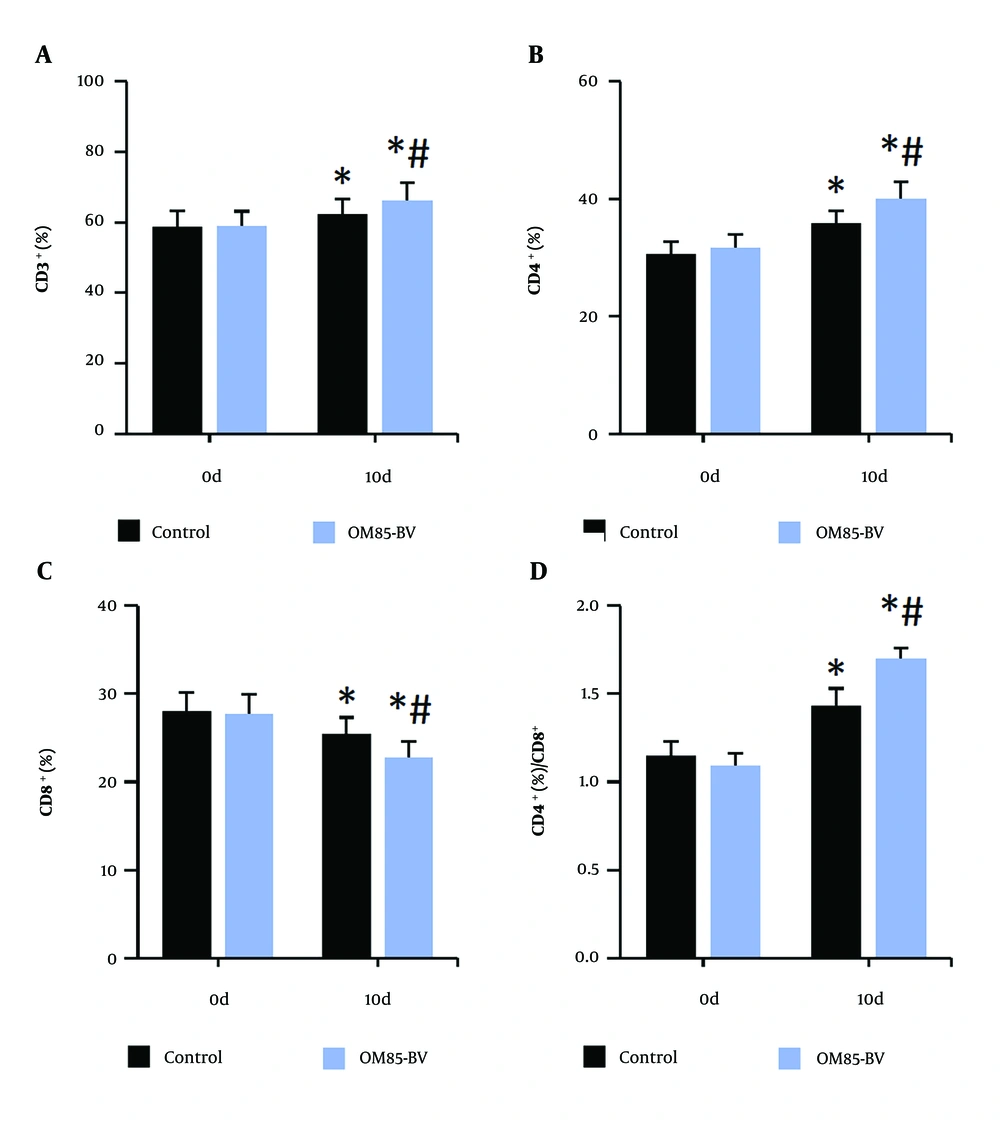
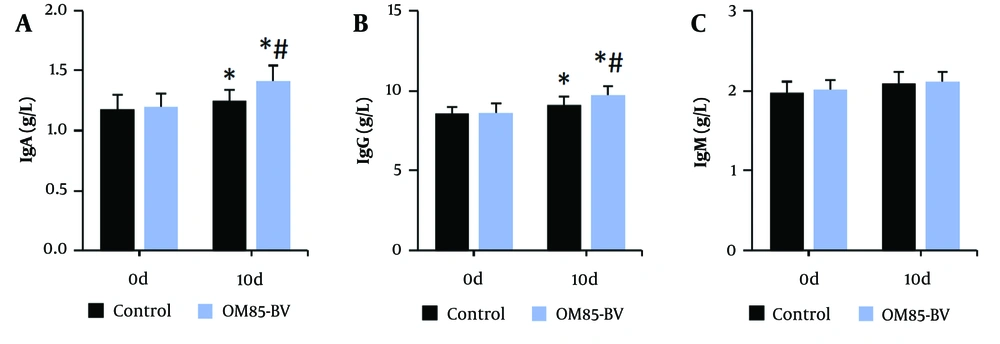
Figure 4 illustrates that, compared to the control group, the levels of CD3+, CD4+, and the CD4+/CD8+ ratio in peripheral blood were significantly increased, while the level of CD8+ was significantly decreased in the OM85-BV group after intervention (all P < 0.05). Figure 5 illustrates that, compared to pre-intervention levels, the levels of IgA and IgG in the peripheral blood of subjects were significantly increased. Additionally, after intervention, the levels of IgA and IgG in the OM85-BV group were significantly higher compared to the control group (all P < 0.05).
4.5. Changes of TLRs/NF-κB Pathway Status in Subjects
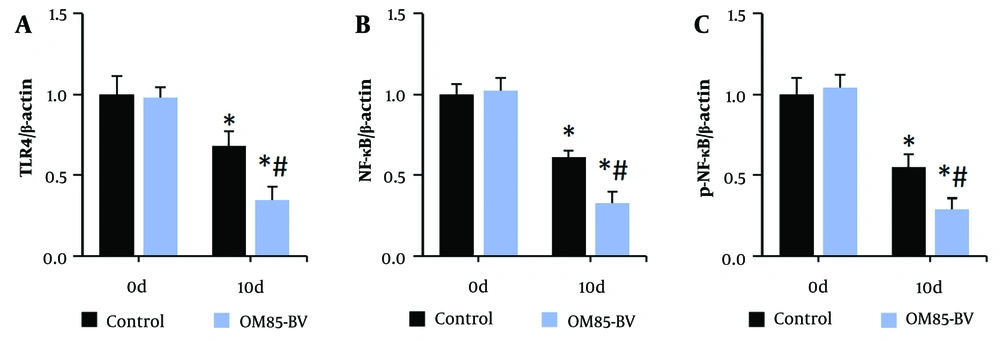
Figure 6 illustrates the protein expression of TLR4, NF-κB, and p-NF-κB in peripheral blood mononuclear cells of subjects. The expression levels were significantly lower after treatment compared to pre-treatment levels. Additionally, the levels in the OM85-BV group were significantly decreased compared to the control group after treatment (all P < 0.05).
4.6. Evaluation of the Efficacy and Safety of the Remedy
Table 1 presents the clinical remedy effects of the subjects. The total clinical effective rate was 80.0% in the control group and 95.0% in the OM85-BV group. The total clinical effective rate of the OM85-BV group was significantly higher compared to the control group (P < 0.05). Table 2 presents the incidence of adverse reactions in subjects. The total incidence of adverse reactions, including nausea/vomiting, abdominal pain/diarrhea, cough, rash, and fatigue, was 13.3% in the control group and 11.7% in the OM85-BV group. There was no significant difference in the total incidence of adverse reactions between the control group and the OM85-BV group (P > 0.05).
| Grouping | Marked Effect | Effective | Invalid | Total Effective Rate |
|---|---|---|---|---|
| Control group (n = 60) | 35 (58.3) | 13 (21.7) | 12 (20.0) | 48 (80.0) |
| OM85-BV group (n = 60) | 46 (76.7) | 11 (18.3) | 3 (5.0) | 57 (95.0) a |
| χ2 | 6.171 | |||
| P | 0.013 |
Contrast of Clinical Efficacy in Children [n (%)]
| Grouping | Nausea/Vomiting | Abdominal Pain/Diarrhea | Cough | Rash | Fatigue | Total AR |
|---|---|---|---|---|---|---|
| Control group (n = 60) | 1 (1.7) | 2 (3.3) | 1 (1.7) | 3 (5.0) | 1 (1.7) | 8 (13.3) |
| OM85-BV group (n = 60) | 3 (5.0) | 2 (3.3) | 1 (1.7) | 1 (1.7) | 0 (0.0) | 7 (11.7) |
| χ2 | 0.076 | |||||
| P | 0.783 |
Contrast of Acute Respiratory in Children [n (%)]
5. Discussion
Children are particularly susceptible to S. pneumoniae infections due to their immature immune systems (12, 13). Antibiotics such as mezlocillin are commonly used to treat these infections by inhibiting the growth of bacterial cell walls (14, 15). The immunomodulator OM85-BV, derived from respiratory pathogens, activates immune cells and enhances the body’s resistance to infections (16, 17). This study aimed to assess the effects of mezlocillin alone versus mezlocillin combined with OM85-BV in treating children with S. pneumoniae lung infections. The results demonstrated that the combined therapy could more rapidly relieve symptoms such as fever, cough, abnormal lung sounds, and abnormal chest X-ray findings. Additionally, it improved lung function without increasing the risk of allergic reactions.
Chest X-rays are widely used in the clinical diagnosis, treatment evaluation, and prognosis prediction of pneumonia. As pneumonia progresses, inflammatory exudates in the lung tissue increase, and chest X-rays reveal signs of inflammatory infiltration and parenchymal lesions, potentially accompanied by an air bronchogram sign and pleural effusion (18-20). Zagolski et al. (21) found that S. pneumoniae counts were significantly reduced in patients with respiratory tract colonization after treatment with bacterial lysates (BL). Similarly, Cao et al. (22) analyzed the effects of OM85-BV in treating recurrent respiratory tract infections and found that it reduced infection rates by improving respiratory immune function, with no significant increase in adverse reactions or wheezing attacks. This study suggests that combining mezlocillin with OM85-BV significantly improves clinical symptoms, chest imaging findings, and lung function in children with S. pneumoniae pulmonary infections, with high efficacy and safety.
It was observed that levels of inflammatory markers, such as CRP, decreased in children's blood after the combined treatment of intravenous mezlocillin and oral OM85-BV. OM85-BV, absorbed in the intestines, activates the immune response, and activated lymphocytes travel to the respiratory tract via the "intestine-lung" axis, thereby enhancing respiratory immunity and resistance to pathogens. Ferrara et al. (23) indicated that bacterial lysates might activate neutrophils, IL-17A, or Caspase-1/11, playing a role in treating pneumococcal pneumonia. Zhu et al. (24) found that bacterial lysates could inhibit the progression of bleomycin-induced pulmonary fibrosis by reducing interferon-γ and IL-4 levels. The combined use of mezlocillin and OM85-BV helps inhibit excessive inflammatory responses.
Lee et al. (25) found that the positive expression rate of IgM and IgG in children with Mycoplasma pneumoniae infections was four times higher compared to healthy children. In this study, the combination of mezlocillin and OM85-BV raised IgA and IgG levels in the peripheral blood of children. These findings suggest that mezlocillin combined with OM85-BV can enhance the immune function of children with S. pneumoniae pulmonary infections by regulating the imbalance of T lymphocyte subsets and increasing immunoglobulin levels, particularly IgA and IgG.
Bacterial infections are a leading cause of pulmonary infections, with S. pneumoniae, Staphylococcus aureus, and Klebsiella pneumoniae being common pathogens in children with community-acquired pneumonia (26). The activation of TLRs/NF-κB signaling promotes the release of inflammatory mediators (27). Tomlinson et al. (28) suggested that S. pneumoniae infections trigger an inflammatory response through TLR signaling, dependent on S. pneumoniae proteins, with NF-κB being activated in macrophages. Wang et al. (29) developed a mouse model of S. pneumoniae infection, showing that survival rates and lung clearance of S. pneumoniae were lower in TLR4-deficient mice, potentially due to the disruption of intestinal microbiota, which may affect drug resistance. Additionally, Chen and Liu (30) found that TNF-α and IL-6 levels increased in bone marrow-derived macrophages following S. pneumoniae infection, accompanied by TLR4/NF-κB activation. Therefore, the TLRs/NF-κB pathway plays a crucial role in regulating pneumococcal-related pulmonary infections.
5.1. Conclusions
In conclusion, the oral administration of bacterial lysates OM85-BV in combination with mezlocillin therapy significantly improves clinical symptoms, lung function, and prognosis in children with S. pneumoniae pulmonary infection. It also enhances immune function, potentially through the inhibition of TLRs/NF-κB activation. The findings from this study offer a new perspective for improving the clinical treatment of S. pneumoniae pulmonary infection in children.