1. Background
In recent decades, opportunistic fungal species have become a cause of fungemia in healthcare settings, resulting in increased mortality and morbidity among patients (1, 2). Candida species play a more important role in fungemia than other fungal species. In fact, candidemia has respectively had the fourth and seventh ranks among nosocomial bloodstream infections in the USA and Europe in the last 20 years (3, 4). In this respect, prophylaxis and antifungal treatments have facilitated the shift toward non-Candida albicans species regardless of geographic location (3, 5). A comprehensive meta-analysis study in 2017 showed that C. parapsilosis is the most common cause of bloodstream infections in Iran (5). In addition, several studies have reported that some less common opportunistic fungi, including Aspergillus, Mucurals, Malassezia, Trichosporon, and Rhodotorula, are responsible for a smaller percentage of fungemia cases (6-). For example, Rhodotorula has been identified as the third most common yeast isolated from blood cultures (BC) (12).
Several factors, including the use of immunosuppressive drugs, treatment with broad-spectrum antibiotics, resistance to common antifungal agents, invasive medical procedures, and the insertion of central venous catheters, are involved in the transformation of yeasts from harmless agents to pathogenic ones (13, 14). In addition, malignancy is one of the most obvious risk factors for fungemia (4). According to a review article, the second predisposing factor for candidemia/fungemia in Iran is malignancy (20%) (5). The highest mortality rate due to invasive fungal infections is reported to be around 80%, of which 30 - 50% is attributed to Candida species in patients with malignancy (4, 15, 16). Since these patients cannot avoid many of the above-mentioned factors, a quick diagnosis can play an important role in their treatment (17). E-test strips and the disc diffusion test (DDT) are simple and rapid diagnostic methods for determining antifungal susceptibility. Today, these tests are frequently utilized because they are simple, their results are consistent with those of the broth microdilution (BMD) reference method, and they require no special equipment (17, 18).
2. Objectives
Due to the discrepancy in the epidemiology of fungemia, the species distribution, the emergence of antifungal drug resistance in different parts of the world, the scarcity of data on fungemia among pediatric patients with malignancies, and the importance of the early diagnosis and treatment of bloodstream infections in these patients, the present study aimed to investigate the prevalence of fungemia and the predominant yeast pathogens and antifungal susceptibility in patients with malignancy and fever over a period of 18 months. The patients were hospitalized in a children’s hospital, a tertiary hematology oncology institute in Ahvaz, Iran. This is the first study in southwestern Iran to investigate fungemia caused by yeast species and antifungal susceptibility in children with malignancy. Hence, its results can be highly beneficial in treating fungemia.
3. Methods
3.1. Study Design and Setting
The present study was a cross-sectional analysis that was conducted with the consent of the parents of the participating children. Patients under the age of 18 (pediatric patients) with malignancy and fever hospitalized in Baghaee Hospital, Ahvaz, were selected for BC from September 2020 to March 2022. Antifungal therapy recipients and positive bacterial samples were excluded from the study. According to the physician’s opinion and the patient’s condition, the treatment was performed as monotherapy or multitherapy. Fungemia is defined as the presence of any fungal species in the blood, whereas fever is described as an oral temperature of ≥ 38.3°C and/or an axillary temperature of ≥ 37.4°C. Candida species were specifically considered as causative agents of fungemia. The demographic data, including the type of underlying disease, risk factors, clinical characteristics, antifungal treatment after diagnosis, and the outcome of the patients, were collected. Patients with positive BC (4-day incubation period) were followed up until a final negative result was obtained. After the treatment and the negative result of BC, if the symptoms reappeared, a re-sampling was done. If BC was positive, it was considered as a separate case.
3.2. Isolation of Yeast
Five to ten mL of fresh whole blood from each patient with malignancy was inoculated into blood culture fluid bottles with S.P.S (Mirmedia, Iran) and immediately sent to the Central Medical Mycology Laboratory of Imam Khomeini Hospital in Ahvaz. After proper aeration, the BC bottles were incubated at 35°C for 4 days. For the recovery of fungal colonies, 50 μL of each positive BC bottle was inoculated on CHROMagar Candida (CHROMagar Candida Company, France) and Sabouraud dextrose agar (SDA) (Merck, Germany). In addition, negative BC bottles were checked each day for up to 14 days. Finally, a direct smear was prepared daily for the detection of fungal elements. With the growth of yeast, the culture was considered positive. Otherwise, the result was reported negative. The colonies were cultured on an SDA medium for purification. A water suspension of each yeast colony was prepared for storage to be recovered during susceptibility testing and molecular identification.
3.3. DNA Extraction and the 21-plex PCR Technique
The genomic DNA of the yeast isolates was extracted from the 48-hour culture as described by Makimura et al. (19). The yeast isolates were identified using the 21-plex PCR technique, which is a novel multiplex PCR assay with three steps. This technique was recently proposed by Arastehfar et al. (15). In summary, the first stage of the 21-plex PCR technique specifically identifies common pathogenic Candida species, the second stage of PCR identifies the less common pathogenic Candida species, and finally, the third stage of PCR detects the non-C. albicans pathogenic yeasts. Five µL of each product and a 100-bp ladder were separated by electrophoresis on a 2% agarose gel. The DNA fragments were stained with red gel, visualized under UV light, and compared with the ladder to determine the size of the fragment.
3.4. Antifungal Susceptibility Testing
Antifungal susceptibility testing (AFST) was performed using both agar-based E-test (Tana Biotech, Iran) and DDT (Cypress Diagnostics, Belgium) methods for 29 of Candida spp. The DDT method was applied to 4 Rhodotorula isolates. A suspension (0.5 McFarland) of each isolate was prepared using a sterile saline solution. Each suspension was streaked in three directions on the surface of the agar plate containing 8.4 g/L RPMI 1640 (Gibco, UK), 2% glucose, and 1.5% agar buffered to PH 7.0 with 0.165 M 3-morpholino-propane-1-sulfonic acid (MOPS) buffer for the E-test strip, while the Mueller-Hinton agar supplemented with 2% glucose and methylene blue stain (0.5 mg/mL) was employed for the DDT. Two reference strains were used to control the process of AFST, namely C. parapsilosis ATCC 22019 and C. krusei ATCC 6258.
The inoculated plates were dried for 15 minutes at ambient temperature. For the E-test, E-test strips containing amphotericin B (AMB), voriconazole (VCZ), posaconazole (PCZ), itraconazole (ICZ), caspofungin (CAS) (0.003∼32 μg/mL), and fluconazole (FLU) (0.016∼256 μg/mL) were placed on the plates, whereas for DDT, paper disks containing AMB (100 µg), FLU (25 µg), VOR (1 µg), ICZ (10 µg), CAS (5 µg), and POS (5 µg) were placed on the plates to determine susceptibility. According to the manufacturer’s manual, the MIC endpoint of each antifungal agent was determined after incubation for 24 and 48 hours at 35 C for the E-test strip. The inhibitory zone diameters were measured after 20 and 24 hours of incubation. The results were interpreted based on the MIC breakpoints of Candida species in 3rd ed. M27M44S from Clinical and Laboratory Standards Institute (CLSI) guidelines and previous studies (20, 21). The MIC of AMB was reported as the lowest drug concentration with 100% growth inhibition. The MICs of FLU, ITR, VOR, POS, and CAS showed 80% growth inhibition compared to positive controls (22).
4. Results
4.1. The Characteristics of the Patients with Malignancy and Fungemia
In this survey, over a period of 18 months (September 2020 to March 2022), 205 blood samples were taken from the study population. Among these samples, 121 (59 %) cases were negative, whereas 48 (23.4%) and 36 (17.6%) cases were positive for bacteremia and fungemia, respectively. The average age of the patients was 8.9 years, ranging from 1 to 14 years. Twenty-eight (77.8%) of these patients were males, while the remaining eight (22.2%) were females. The incidence of fungemia was 56.8% higher in males than in females. Thirty-three patients (91.7%) were hospitalized for an average period of 30.5 days. Although none of the patients received primary antifungal prophylaxis, they were exposed to antibiotics.
It was also found that the overall prevalence of fungemia was higher in acute lymphoblastic leukemia (ALL) patients than in those with other malignancies. The main outcome observed in this study was the discharge of 29 patients (78%). Three of the patients (8%) were deceased, and 5 of them (13.5%) were referred to another hospital. Candidemia with C. parapsilosis, C. albicans, and C. orthopsilosis was diagnosed in the three deceased patients on the 2nd, 20th, and 20th days of hospitalization, respectively, and they received antifungal therapy. After antifungal therapy, the BCs of these patients were negative, so their death after 30 days was not associated with candidemia. It should be noted that no mixed fungemia or candidemia was detected. Nevertheless, candidemia caused by C. orthopsilosis was observed in a three-year-old patient. Two months after treatment, the patient was diagnosed with candidemia caused by C. albicans.
4.2. The Identification of the Yeasts Isolated from Blood Culture Bottles by 21-plex PCR
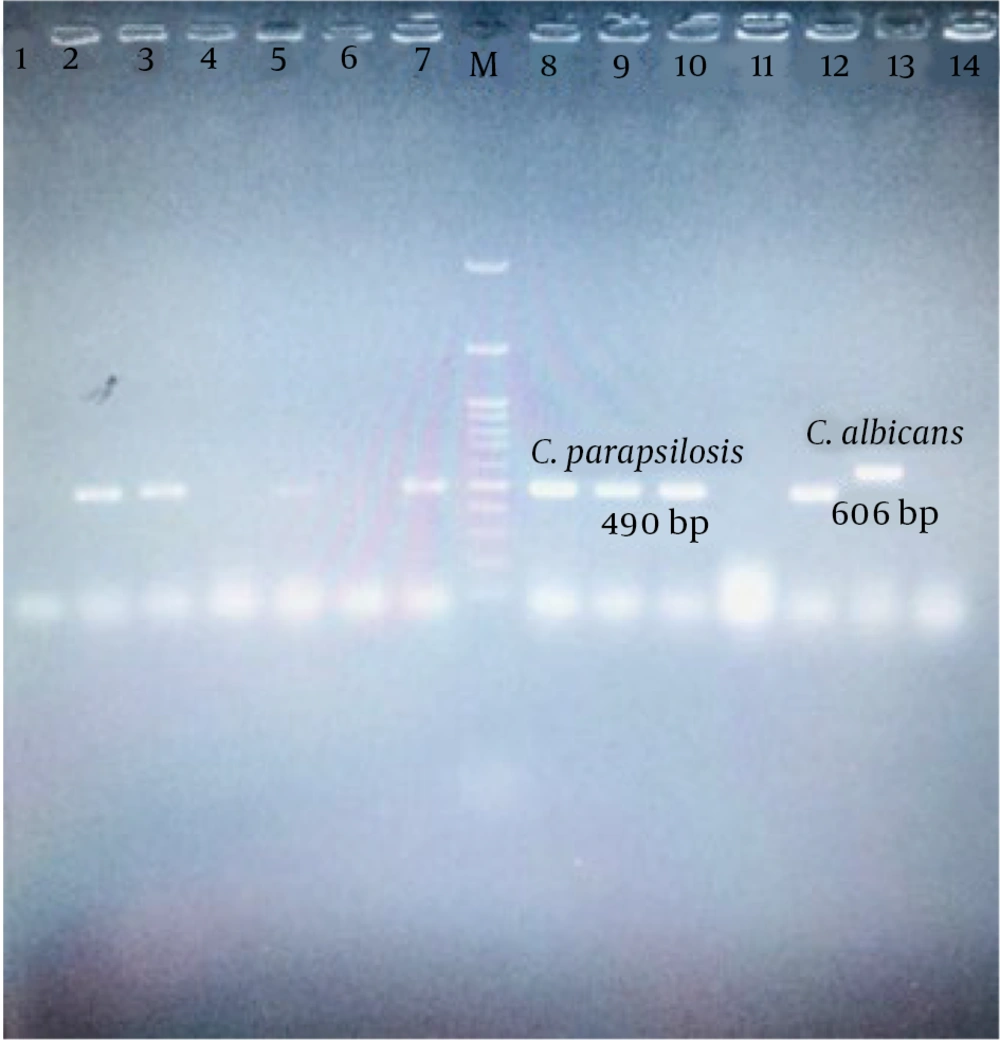
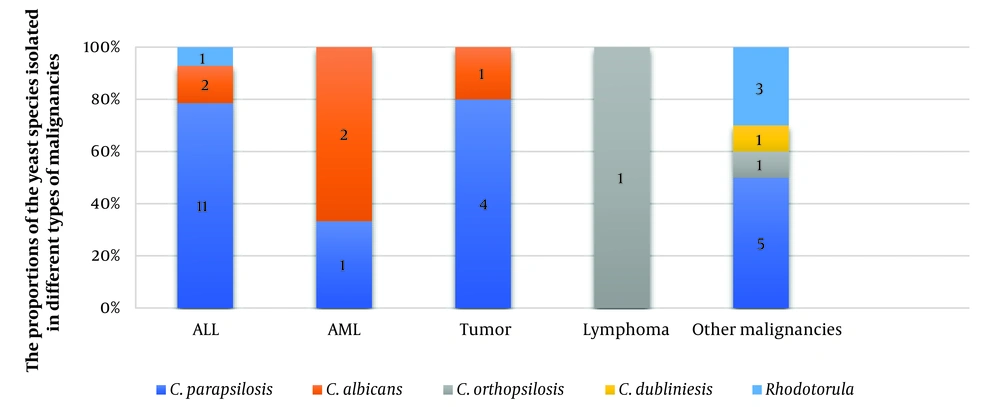
Thirty-six yeast isolates from patients with malignancy-associated fungemia (17.6%) were identified by 21-plex PCR. Among the isolated yeasts, non-C. albicans species were present in 27 cases (75%), C. albicans in 5 cases (13.9 %), and Rhodotorula spp. in 4 cases (11.1%). The results of phenotypic were consistent with the 21-plex PCR method. The data of 36 patients with malignancy-associated fungemia are shown in Table 1. A total of 32 (88.9%) cases of candidemia with a predominance of non-C. albicans species, as well as 4 (11.1%) cases of infection by Rhodotorula isolates, were diagnosed by the 21-plex PCR Technique (Figure 1). Three cases of candidemia were not included in the study due to the contamination of the medium and the inability to recover the causative agent. Candida parapsilosis was detected as the most frequent cause of candidemia in patients with malignancy. Besides, the two rare species of C. orthopsilosis and C. dubliniensis were the causative agents of three (9.4%) cases of candidemia. (Figure 2). Eleven (32.4%) samples were taken from both the port and vein simultaneously, of which 10 cases (90.9%) showed infection with the same Candida species, and only one case showed infection with two different Candida species. A mixed infection was not observed.
| Variables | No (%) |
|---|---|
| Gender | |
| Male | 28 (77.8) |
| Female | 8 (22.2) |
| Underlying factors | |
| ALL | 14 (38.9) |
| Tumor | 5 (13.8) |
| AML | 3 (8.3) |
| Lymphoma | 2 (5.5) |
| Other malignancies | 12 (33.5) |
| Risk factors | |
| Central venous catheter | 36 (100) |
| Receiving antibiotics | 36 (100) |
| Antifungal prophylaxis | - |
| Clinical characteristics | |
| Neutropenia a | 9 (24.3) |
| Thrombocytopenia b | 17 (46) |
| CRP positive | 16 (43.2) |
| Mean ESR/range | 53.3/1-133 |
| Mean Hb/range | 10.2/6-14.7 |
| Fever | 36 (100) |
| Treatment after diagnosis | |
| Caspofungin | 16 (44.4) |
| Amphotericin B | 14 (38.9) |
| Fluconazole | 11 (30.5) |
| Voriconazole | 8 (22.2) |
| Itraconazole | 1 (2.7) |
| Monotherapy | 16 (44.4) |
| Combined treatment | 13 (36.1) |
| Not receiving antifungal agents (removal of the catheter) | 4 (11.1) |
| Outcome | |
| Discharge | 29 (78) |
| Referral | 5 (13.5) |
| Death | |
| Death > 30 days after antifungal therapy | 3 (8) |
| Death < 30 days after antifungal therapy | 0 |
| Persistent fungemia | 0 |
| Length of stay | |
| Short (< 7 days) | 3 (8.3) |
| Long (≥ 7 days) | 33 (91.7) |
| Fungal species | |
| C. albicans | 5 (13.9) |
| Non-C. albicans spp. | 27 (75) |
| Rhodotorula spp. | 4 (11.1) |
The Clinical-Epidemiological Characteristics of 37 Cases of Fungemia (Diagnosed by Blood Culture) with Malignancy
4.3. The Results of Antifungal Susceptibility
Most yeasts isolated by the above-mentioned methods were susceptible to the six antifungal agents under study (Table 2). Candida parapsilosis had the highest resistance to CAS (7; 33.3%), followed by ICZ (5; 23.8%) and AMB (2; 9.6%). ICZ resistance was also observed in a species of C. orthopsilosis (1; 50%). Other species were susceptible or dose-dependently susceptible to the six antifungal agents. There was no known breakpoint for PCZ. Hence, the MIC value and the inhibition zone diameter were recorded. Candida species mostly showed low MICs for PCZ (Table 3).
| Species (N) and Drug | E-test | Disc Diffusion | ||||
|---|---|---|---|---|---|---|
| S (%) | I/SDD (%) | R (%) | S (%) | I/SDD (%) | R (%) | |
| C. parapsilosis (21) | ||||||
| AMP | 19 (90.4) | 2 (9.6) | 18 (85.7) | 3 (14.3) | ||
| FCZ | 21 (100) | 20 (95.2) | 1 (4.8) | |||
| ICZ | 14 (66.7) | 2 (9.5) | 5 (23.8) | 14 (66.7) | 7 (33.3) | |
| VCZ | 20 (95.2) | 1 (4.8) | 20 (95.2) | 1 (4.8) | ||
| CAS | 14 (66.7) | 7 (33.3) | 14 (66.7) | 7 (33.3) | ||
| PCZ | - | - | - | - | - | - |
| C. albicans (5) | ||||||
| AMP | 5 (100) | 5 (100) | ||||
| FCZ | 5 (100) | 5 (100) | ||||
| ICZ | 5 (100) | 5 (100) | ||||
| VCZ | 4 (80) | 1 (20) | 3 (60) | 2 (40) | ||
| CAS | 5 (100) | 5 (100) | ||||
| PCZ | - | - | - | - | - | |
| C. orthopsilosis (2) | ||||||
| AMP | 2 (100) | 2 (100) | ||||
| FCZ | 2 (100) | 2 (100) | ||||
| ICZ | 1 (50) | 1 (50) | 1 (50) | 1 (50) | ||
| VCZ | 2 (100) | 2 (100) | ||||
| CAS | 2 (100) | 2 (100) | ||||
| PCZ | - | - | - | - | - | |
| C. dubliniesis (1) | ||||||
| AMP | 1 (100) | 1 (100) | ||||
| FCZ | 1 (100) | 1 (100) | ||||
| ICZ | 1 (100) | 1 (100) | ||||
| VCZ | 1 (100) | 1 (100) | ||||
| CAS | 1 (100) | 1 (100) | ||||
| Rhodotorula spp (4) | ||||||
| AMP | - | - | - | 4 (100) | ||
| FCZ | - | - | - | 4 (100) | ||
| ICZ | - | - | - | 4 (100) | ||
| VCZ | - | - | - | 4 (100) | ||
| CAS | - | - | - | 4 (100) | ||
The In-vitro Activity Data of Five Antifungal Agents for 33 Clinical Isolates Using the E-test and Disk Diffusion Methods
| Species | Minimum Inhibitory Concentration Values (μg/mL) | Range of Zone of Inhibition (mm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ||
| C. parapsilosis | 6 | 8 | 5 | 2 | 18 - 30 | |||||||
| C. albicans | 3 | 1 | 1 | 18 - 28 | ||||||||
| C. orthopsilosis | 2 | 20 - 23 | ||||||||||
| C. dubliniesis | 1 | 20 | ||||||||||
| Rhodotorula spp. | - | - | - | - | - | - | - | - | - | - | - | 18 - 38 |
The Antifungal Susceptibility Data of 34 Clinical Isolates Using E-test and Disk Diffusion Methods for Posaconazole
5. Discussion
Fungemia is a major life-threatening problem in immunocompromised patients, including those with malignancy (4, 23). Meanwhile, candidemia (with an approximate incidence of 35%) has overtaken other fungemia agents with the dominance of non-C. albicans species over albicansCandida species (1, 13). In this study, due to the limitations of phenotypic methods, all isolated yeast species were identified by 21-plex PCR. According to Arastehfar et al., this method is a suitable diagnostic strategy with high speed and sufficient accuracy for the detection and differentiation of yeast species, and its results are consistent with the results of the MALDI-TOF MS technique (15). By this method, we detected the rare C. orthopsilosis (n = 2) species. At first, it was deemed to be C. parapsilosis by phenotypic methods; however, 21-plex PCR confirmed the diagnosis of C. parapsilosis.
We demonstrated that non-C. albicans species were the main etiological agents of candidemia. This is in line with several investigations conducted in Iran and the world regarding the shift of candidemia towards non-C. albicans species (13, 16, 24). In our study, among non-C. albicans species (75%), C. parapsilosis was the main causative agent of candidemia in children, which is consistent with previous studies (24-26). However, in other articles, the prevalence of other non-C. albicans species has been reported (1, 4, 27). This disparity may be a result of many factors, including medical practice, patient age, underlying diseases, prophylaxis, geographic distribution, and even actions and reactions that remain unclear.
Since none of our patients had received antifungal prophylaxis, the low susceptibility to azoles and echinocandins was not subject to discussion. However, some research revealed a close relationship between decreased susceptibility to azoles and echinocandins and previous exposure to antifungal agents in patients with candidemia (28, 29). Due to the high affinity of C. parapsilosis for foreign materials (such as catheters) frequently used for patients with malignancies as well as its growth in liquid nutrition medium (23), regional factors probably play a more important role in the infections caused by C. parapsilosis. In addition, although some of the patients had taken antibiotics, had catheters, and had received intravenous nutrition, no association was found between these risk factors and candidemia or infection with a specific fungal species. In the present study, it was found that 100% of the patients had a central venous catheter. It is noteworthy that some studies have associated the presence of catheters with fungemia (23, 28, 30, 31).
Three (9.4%) candidemia cases caused by infection with uncommon species were detected. Although this finding is consistent with the report of Tsai et al., the emergence of uncommon species in our study was independent of antifungal prophylaxis, so 66.7% of these patients (both infected with C. orthopsilosis) recovered with longer antifungal therapy (13). Candidemia is a risk factor for mortality in patients with malignancy. However, in our follow-up, no mortality was recorded < 30 days after antifungal treatment. Previous studies support the possibility that patients infected with C. parapsilosis have a lower mortality rate than those infected with other Candida species, especially C. albicans (26, 32). On the other hand, BCs were performed on patients with even minimal symptoms during the COVID-19 pandemic, leading to the early diagnosis and treatment of candidemia. However, though the BCs of the three deceased patients were negative for fungi, they died 30 days after antifungal treatment.
These deaths were probably caused by the underlying diseases and their severity. Four cases of fungemia due to Rhodotorula spp. were found. With the removal of the catheter, their conditions improved, and they did not receive antifungal treatment. We hypothesized that it was a fungal colonization that was resolved with early detection. In the present study, the E-test and DDT methods were used because they are easy, fast, and inexpensive. In addition, the results of these methods agree with those of the BMD reference method for Candida spp., indicating their reliability (18). In general, the results of the E-test and DDT methods were very similar with respect to the Candida isolates. However, there was a slight difference between the two methods in some Candida strains regarding susceptibility and/or dose-dependent susceptibility to antifungal agents (Table 2). In line with our results, the findings of Kumar et al. showed a good correlation between the inhibitory zone diameter and the MICs of the E-test and BMD tests and indicated a slight difference between the methods (18). Among the identified species, the highest level of variation in the results between the two methods was related to the dose-dependent susceptible strains of C. parapsilosis. In the present study, the C. parapsilosis isolates (33.3%) showed high resistance to CAS, though it is a known fungicide and the first line of treatment for Candida infections (33). In addition, the isolates in the present study showed 28.6% and 38.1% resistance to ICZ based on the results of the E-test and DDT methods, respectively. Interestingly, three C. parapsilosis isolates were found to be resistant to both ICZ and CAS. However, contrary to our expectations, they were susceptible to other azoles with very low MICs.
Candida albicans, the second most common species in this study, showed no resistance to any of the antifungal agents. These results were consistent with the Gonzalez-Lara report (16). Overall, all Candida isolates were susceptible to FCZ, indicating its superior performance over AMP, ICZ, VCZ, or CAS. Several articles have reported the increased resistance of non-C. albicans species to azoles (27, 28). In addition, similar to our findings, some papers have reported the low resistance percentage of Candida spp. to AMP (22, 34). Moreover, using DDT, the AFST results of the Rhodotorula isolates showed their susceptibility to AMP and ITR as well as their high resistance to FLC, CAS, and VRC, which is in agreement with the study of Seifi et al. (21).
Since there is no known breakpoint for the responses of Candida and Rhodotorula spp. to PCZ, it is not possible to interpret the isolates as "resistant" or "susceptible" to this antifungal agent (35). However, unlike Rhodotorula spp., Candida spp. showed low and acceptable MIC values for PCZ. In agreement with our results, Ahmed et al. and Khumdee et al. reported the resistance of Rhodotorula spp. and the susceptibility of Candida spp. to PCZ, respectively (35, 36). Although the results of in-vitro and in vivo susceptibility patterns do not completely match each other, knowledge of in-vitro antifungal susceptibility patterns is an initial and appropriate strategy before starting treatment. In this way, an inappropriate antifungal agent can be rejected, the chance of treatment success increases, and the risk of mortality decreases.
This is especially the case in patients with malignancy. In the present study, the treatment of candidemia for the patients was in the form of monotherapy or multitherapy based on the consensus results of the E-test and DDT. Furthermore, the dosage of the antifungal agent was determined according to the physician's opinion based on the patient's weight and clinical conditions. Fortunately, the therapy was successful, and no increase in the length of the treatment period (except in two cases of C. orthopsilosis) or mortality was observed. The present study had some limitations. First, we could not fully control the patients' conditions as the duration of hospitalization was reduced to a minimum due to the COVID-19 pandemic. Second, fungemia was diagnosed using a conventional BC method since we did not have access to the BACTEC or other modern systems. The BC method has a lower susceptibility than the more modern systems and may have caused false-negative results and underreporting.
5.1. Conclusions
Despite the deficiency of the immune system in malignant patients, non-C. albicans spp. (C. parapsilosis) were the most frequent cause of fungemia, and the other opportunistic yeasts were insignificant in this respect. Overall, no mortality was observed in this study, which is justified by the high prevalence of C. parapsilosis among the patients and early diagnosis. In addition to timely diagnosis and awareness of antifungal susceptibility patterns, the management of treatment and its follow-up should be given serious attention so that a reduction in the incidence of these infections can be witnessed. Based on the results of our study, none of the Candida species isolates showed resistance to FCZ.