1. Background
Children are significantly impacted by annual influenza epidemics, experiencing the highest attack rates among all age groups. Infants and young children are frequently hospitalized due to influenza-related illnesses, classifying them as a high-risk group (1, 2). Influenza viruses belong to the Orthomyxoviridae family and are enveloped viruses. Based on the antigenic differences of the nucleocapsid protein (NP) and matrix protein (MP) within the virus, they are classified into four types (3). Currently, the viruses causing seasonal influenza epidemics are the H1N1 and H3N2 subtypes of type A, and the Victoria and Yamagata lineages of type B. Although both influenza A and B viruses can lead to hospitalization and various complications in children, there are differences in disease severity and the age of the infected children. Children infected with influenza A are generally younger than those infected with influenza B (4). Additionally, the morbidity and mortality rates of influenza A are higher compared to influenza B (4).
Specifically, the clinical characteristics of influenza B infection in children and its comparisons with the influenza A subtypes H1N1 and H3N2 are topics of ongoing research. These influenza strains are associated with a variety of clinical presentations, and understanding these differences could potentially influence treatment strategies. However, the extent and nature of these differences, particularly in pediatric patients, remain largely unexplored. There have been reports suggesting that influenza virus co-infections with other respiratory pathogens (3, 5, 6) may add another layer of complexity. When considering the implications of co-infections in pediatric patients involving influenza B, influenza A/H1N1, and A/H3N2, these co-infections could potentially lead to more severe disease progression (6, 7). However, the specific dynamics of these co-infections and their impact on disease severity are not well-researched.
2. Objectives
This study aims to compare the clinical presentations in pediatric patients infected with influenza B versus influenza A subtypes H1N1 or H3N2 and to investigate whether co-infections with these strains and other respiratory pathogens worsen disease severity. By achieving these objectives, we hope to provide a more comprehensive understanding of these infections, potentially paving the way for improved treatment strategies.
3. Methods
3.1. Patient Selection
In a retrospective study conducted from 2022 to 2023, children aged 1 to 14 who were hospitalized with acute respiratory infections at the Maternal and Child Health Hospital of Hubei province were assessed. Disease severity across infection groups was evaluated based on predefined criteria such as pneumonia incidence, mechanical ventilation use, pediatric intensive care unit (PICU) admission rate, and duration of hospital stay. The analysis included demographic, epidemiological, diagnostic, and laboratory data.
3.2. Respiratory Pathogen Detection
All pathogen detection data were sourced from electronic medical records. Respiratory virus detection was performed using PCR, targeting influenza A/H1N1, H3N2, influenza B, human rhinovirus (HRV), respiratory syncytial virus (RSV), human parainfluenza virus (HPIV), human coronaviruses (HCoV), human adenovirus (HAdV), human metapneumovirus (hMPV), human bocavirus (HBoV), Mycoplasma pneumoniae, and Chlamydia. Microbial culture and identification were conducted within 24 hours of admission, following the hospital's standard diagnostic procedures.
3.3. Exclusion Criteria
The exclusion criteria for participants in the study were: (1) chronic pulmonary disease, aspiration pneumonia, or interstitial lung disease; (2) compromised immunity or the use of immunosuppressive drugs; (3) suspected hospital-acquired or fungal infections; (4) insufficient clinical information; and (5) confirmed cases of COVID-19 infection in children.
3.4. Statistical Analysis
Statistical analyses were performed using SPSS version 21.0. Frequency comparisons between groups were conducted using the chi-square or Fisher’s exact test, while comparisons of mean values were performed using the independent sample t-test. A P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Demographic Distribution by Influenza Virus Type and Subtype
A total of 1,380 pediatric patients with influenza virus infection were included in the study, consisting of 343 cases of Influenza A/H1N1, 678 cases of Influenza A/H3N2, and 359 cases of Influenza B. Among cases of single infections, the majority of children infected with Influenza A (H1N1 and H3N2) were aged 3 - 5 years, accounting for 49.5% and 48.5%, respectively. In contrast, Influenza B predominantly affected children aged 6 years and older, with a proportion of 43.9%. There were no significant differences in gender ratios across the groups (Table 1).
| Demographics | Mono-infection | Co-infection | ||||||
|---|---|---|---|---|---|---|---|---|
| Influenza B (n = 321) | A/H1N1 (n = 331) | A/H3N2 (n = 597) | P-Value | Influenza B (n = 38) | A/H1N1 (n = 12) | A/H3N2 (n = 81) | P-Value | |
| Age (y) | < 0.001 | 0.039 | ||||||
| 1 - 2 | 76 (23.7) | 60 (18.1) | 163 (27.3) | 17 (44.7) | 3 (25.0) | 15 (18.5) | ||
| 3 - 5 | 104 (32.4) | 164 (49.5) | 290 (48.5) | 8 (21.1) | 5 (41.7) | 33 (40.7) | ||
| ≥ 6 | 141 (43.9) | 107 (32.3) | 144 (24.1) | 13 (34.2) | 4 (33.3) | 33 (40.7) | ||
| Gender | 0.340 | 0.371 | ||||||
| Male | 183 (57.0) | 193 (58.3) | 368 (61.6) | 23 (60.5) | 5 (41.7) | 51 (63.0) | ||
| Female | 138 (43.0) | 138 (41.7) | 229 (38.4) | 15 (39.5) | 7 (58.3) | 30 (37.0) | ||
a Values are expressed as No. (%).
4.2. Comparative Clinical Characteristics of Single Infections: Influenza B vs. Influenza A/H1N1 and A /H3N2
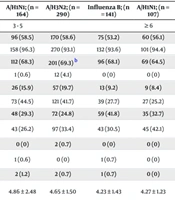
In cases of mono-infection among children aged six and older, the incidence of pneumonia was significantly higher in those infected with Influenza A/H3N2 compared to Influenza A/H1N1 (P = 0.007) and Influenza B (P = 0.013). Additionally, the duration of hospitalization for A/H3N2 patients was longer than for those with the other two strains (H1N1: P = 0.046; B: P = 0.044), as detailed in Table 2. Within the same age group, differences in laboratory results were observed between children with single infections of influenza A and B (Table 2). Children with influenza A/H1N1 exhibited a significantly higher proportion of lymphocytes compared to those with influenza A/H3N2 or influenza B, regardless of age (P < 0.001). Additionally, for children aged three and older, platelet counts were significantly higher in those with influenza A/H1N1 compared to those with influenza B (P < 0.001). The rate of antiviral drug usage among children infected with influenza A was significantly higher than that among children infected with influenza B across all age groups (P < 0.001).
| Clinical Information | Influenza B (n = 76) | A/H1N1 (n = 60) | A/H3N2 (n = 163) | Influenza B (n = 104) | A/H1N1 (n = 164) | A/H3N2 (n = 290) | Influenza B (n = 141) | A/H1N1 (n = 107) | A/H3N2 (n = 144) |
|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 1 - 2 | 3 - 5 | ≥ 6 | ||||||
| Gender (male) | 48 (63.2) | 37 (61.7) | 106 (65) | 60 (57.7) | 96 (58.5) | 170 (58.6) | 75 (53.2) | 60 (56.1) | 92 (63.9) |
| Fever | 71 (93.4) | 57 (95) | 154 (94.5) | 101 (97.1) | 158 (96.3) | 270 (93.1) | 132 (93.6) | 101 (94.4) | 135 (93.8) |
| Cough | 46 (60.5) | 35 (58.3) | 79 (48.5) | 61 (58.7) | 112 (68.3) | 201 (69.3) b | 96 (68.1) | 69 (64.5) | 102 (70.8) |
| Wheezing | 3 (3.9) | 2 (3.3) | 3 (1.8) | 4 (3.8) | 1 (0.6) | 12 (4.1) | 0 (0) | 0 (0) | 1 (0.7) |
| Rales | 17 (22.4) | 14 (23.3) | 42 (25.8) | 19 (18.3) | 26 (15.9) | 57 (19.7) | 13 (9.2) | 9 (8.4) | 27 (18.8) b,c |
| Pneumonia | 29 (38.2) | 24 (40.0) | 55 (33.7) | 39 (37.5) | 73 (44.5) | 121 (41.7) | 39 (27.7) | 27 (25.2) | 60 (41.7) b,c |
| Bronchitis | 16 (21.1) | 11 (18.3) | 34 (20.9) | 26 (25.0) | 48 (29.3) | 72 (24.8) | 59 (41.8) | 35 (32.7) | 45 (31.3) |
| Upper respiratory infection | 31 (40.8) | 25 (41.7) | 74 (45.4) | 39 (37.5) | 43 (26.2) | 97 (33.4) | 43 (30.5) | 45 (42.1) | 39 (27.1) |
| Oxygen support | 1 (1.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Mechanical ventilation | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) | 1 (0.6) | 0 (0) | 1 (0.7) | 0 (0) | 0 (0) |
| PICU admission | 1 (1.3) | 1 (1.7) | 1 (0.6) | 0 (0) | 2 (1.2) | 2 (0.7) | 1 (0.7) | 0 (0) | 0 (0) |
| Hospitalization length of stay (d) | 4.79 ± 1.75 | 4.88 ± 1.94 | 4.46 ± 1.67 | 4.43 ± 1.42 | 4.86 ± 2.48 | 4.65 ± 1.50 | 4.23 ± 1.43 | 4.27 ± 1.23 | 4.62 ± 1.44 b,c |
| WBC, × 109/L | 6.44 ± 3.40 | 5.66 ± 2.47 | 6.65 ± 2.88 | 6.00 ± 3.51 | 5.22 ± 2.20 b | 6.73 ± 3.61 | 5.50 ± 3.03 | 5.18 ± 3.17 | 5.78 ± 2.75 |
| Lymphocytes (%) | 45.42 ± 21.40 | 59.19 ± 23.82 b | 36.19 ± 21.03 b,c | 36.21 ± 18.54 | 53.52 ± 20.41 b | 35.42 ± 21.11 c | 32.43 ± 17.03 | 44.97 ± 20.65 b | 33.59 ± 20.94 c |
| PLT, × 109/L | 210.24 ± 80.99 | 237.65 ± 102.92 | 242.39 ± 85.51 b | 209.82 ± 68.64 | 252.18 ± 93.02 b | 249.49 ± 97.69 b | 201.72 ± 67.96 | 241.65 ± 79.32 b | 228.52 ± 63.27 b |
| ALT, U/L | 19.00 ± 11.05 | 20.09 ± 18.89 | 17.44 ± 8.46 | 16.63 ± 13.66 | 16.86 ± 20.68 | 13.86 ± 6.06b | 16.97 ± 17.50 | 22.21 ± 45.47 | 14.44 ± 67.6 |
| AST, U/L | 52.99 ± 21.72 | 48.24 ± 29.09 | 44.47 ± 12.86 b | 46.11 ± 26.64 | 43.25 ± 22.28 | 38.21 ± 15.91 b | 41.85 ± 31.69 | 41.29 ± 32.95 | 36.26 ± 19.34 |
| CK-MB, U/L | 34.07 ± 16.10 | 33.35 ± 28.17 | 32.06 ± 17.39 | 29.95 ± 17.47 | 31.03 ± 28.13 | 29.67 ± 20.51 | 26.27 ± 16.38 | 24.51 ± 20.29 | 24.33 ± 15.56 |
| LDH, U/L | 361.08 ± 73.03 | 327.60 ± 120.07 | 337.17 ± 83.99 b | 310.35 ± 72.71 | 307.48 ± 117.70 | 307.76 ± 81.97 | 287.85 ± 79.95 | 270.62 ± 64.10 | 267.92 ± 58.02 b |
| Use of antiviral drugs | 26 (34.2) | 42 (70.0) b | 106 (65.0) b | 32 (30.8) | 112 (68.3) b | 165 (56.9) b,c | 46 (32.6) | 67 (62.6) b | 92 (63.9) b |
Abbreviation: PICU, pediatric intensive care unit.
a Values are expressed as No. (%) or mean ± SD.
b P < 0.05, compared with influenza B.
c P < 0.05, compared with influenza A/H1N1.
4.3. Co-pathogens Detected in Influenza Virus Infections
The co-infection rate of Influenza A/H1N1 is 3.5%, which is significantly lower than the 11.9% for influenza A/H3N2 (P < 0.001) and 10.6% for influenza B (P < 0.001). In the 12 cases of co-infection with influenza A/H1N1, all co-infecting pathogens were viruses. In the 81 cases of Influenza A/H3N2 co-infections, 52 (64.2%) were co-infected with viruses, 4 (4.9%) with bacteria, 20 (24.7%) with M. pneumoniae, and 5 (6.2%) involved multiple co-infections (with three or more pathogens). Among the 38 cases of Influenza B co-infections, 29 (76.3%) were co-infected with viruses, 3 (7.9%) with bacteria, 3 (7.9%) with M. pneumoniae, and 3 (7.9%) involved multiple co-infections. Details of the co-infecting pathogens are provided in Table 3. The respiratory viruses that primarily co-infected with influenza include HRV, RSV, HPIV, HAdV, hMPV, HCoV, and HBoV. The co-infected bacteria were mainly Streptococcus pneumoniae, Haemophilus influenzae, and Pseudomonas aeruginosa, with S. pneumoniae being the most common.
| Influenza B (n = 38) | A/H1N1 (n = 12) | A/H3N2 (n = 81) | ||||||
|---|---|---|---|---|---|---|---|---|
| With Virus (n = 29) | With Bacteria (n = 3) | With M. pneumoniae (n = 3) | With More Than Two Pathogens (n = 3) | With Virus (n = 12) | With Virus (n = 52) | With Bacteria (n = 4) | With M. pneumoniae (n = 20) | With More Than Two Pathogens (n = 5) |
| HRV | S. pneumoniae | RSV + HAdV | HRV | HRV | S. pneumoniae | HAdV + M. pneumoniae | ||
| RSV | P. aeruginosa | RSV + HRV | RSV | RSV | H. influenzae | HPIV + M. pneumoniae | ||
| HAdV | M. Pneumoniae + HAdV | HAdV | HAdV | HPIV+HRV | ||||
| HPIV | HPIV | HPIV | M. Pneumoniae + S. pneumoniae | |||||
| hMPV | hMPV | |||||||
| HCoV | HBoV | |||||||
Abbreviations: HRV, human rhinovirus; RSV, respiratory syncytial virus; HPIV, human parainfluenza virus; HCoV, human coronaviruses; HAdV, human adenovirus; hMPV, human metapneumovirus; HBoV, human bocavirus; M. pneumonia, Mycoplasma pneumonia; S. pneumoniae, Streptococcus pneumoniae; H. influenzae, Haemophilus influenzae; P. aeruginosa, Pseudomonas aeruginosa.
4.4. Comparative Clinical Characteristics: Single vs. Co-infections in Pediatric Patients with Influenza B, A/H1N1, and A/H3N2
There were significant differences in the clinical manifestations between the mono-infection and co-infection groups of influenza B and influenza A/H3N2. Patients with co-infections had a significantly higher incidence of pneumonia (B: P = 0.003; H3N2: P < 0.001) and longer hospital stays (B: P = 0.005; H3N2: P = 0.023) compared to those with mono-infections, as shown in Table 4. Additionally, individuals with co-infections of influenza A/H3N2 had a higher likelihood of being admitted to the PICU than those with mono-infections (P = 0.024).
| Clinical Information | Influenza B; Mono-infection(n = 321 | Co-infection(n = 38 | A/H1N1; Mono-infection(n = 331 | Co-infection(n = 12 | A/H3N2; Mono-infection (n = 597) | Co-infection (n = 81) |
|---|---|---|---|---|---|---|
| Clinical presentation | ||||||
| Fever | 304 (94.7) | 36 (94.7) | 316 (95.5) | 12 (100) | 559 (93.6) | 78 (96.3) |
| Cough | 203 (63.2) | 31 (81.6) b | 216 (65.3) | 6 (50.0) | 382 (64.0) | 63 (77.8) b |
| Wheezing | 7 (2.2) | 3 (7.9) | 3 (0.9) | 0 (0) | 16 (2.7) | 4 (4.9) |
| Rales | 49 (15.3) | 11 (28.9) b | 49 (14.8) | 1 (8.3) | 126 (21.1) | 25 (30.9) b |
| Diagnosis | ||||||
| Pneumonia | 107 (33.3) | 22 (57.9) b | 124 (37.5) | 4 (33.3) | 236 (39.5) | 54 (66.7) b |
| Bronchitis | 101 (31.5) | 7 (18.4) | 94 (28.4) | 2 (16.7) | 151 (25.3) | 15 (18.5) |
| Upper respiratory infection | 113 (35.2) | 9 (23.7) | 113 (34.1) | 6 (50.0) | 210 (35.2) | 12 (14.8) b |
| Treatment | ||||||
| Oxygen support | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 2 (0.3) | 2 (2.5) |
| Mechanical ventilation | 2 (0.6) | 0 (0) | 1 (0.3) | 0 (0) | 0 (0) | 1 (1.2) |
| PICU admission | 2 (0.6) | 1 (2.6) | 3 (0.9) | 0 (0) | 3 (0.5) | 3 (3.7) b |
| Hospitalization length of stay (d) | 4.43 ± 1.52 | 5.16 ± 1.37 b | 4.67 ± 2.06 | 4.75 ± 1.60 | 4.59 ± 1.53 | 5.14 ± 2.05 b |
| Use of antiviral drugs | 104 (32.4) | 14 (36.8) | 221 (66.8) | 11 (91.7) | 363 (60.8) | 52 (64.2) |
Abbreviation: PICU, pediatric intensive care unit.
a Values are expressed as No. (%) or mean ± SD.
b P < 0.05.
5. Discussion
Existing literature presents differing opinions on the clinical characteristics and severity of influenza A and B, with some experts suggesting similarities, while others argue that certain strains may cause more severe illness with unique features (8-12). Our study revealed that children aged 6 and older infected with influenza A/H3N2 had a pneumonia rate of 41.7%, significantly higher than the rates for influenza A/H1N1 (25.2%) and influenza B (27.7%). Additionally, the average hospital stay for these children was 4.62 days, longer than the average stays for influenza A/H1N1 (4.27 days) and influenza B (4.23 days). For children aged one to five years, the differences in pneumonia incidence or hospital stay duration were insignificant among the three viruses, despite minor clinical and lab variations. The frequent antigenic drift in the Influenza A/H3N2 virus may account for its increased virulence compared to H1N1 and influenza B (13). Moreover, age is a critical factor when assessing influenza in children, as immune responses and their intensity can vary significantly with age.
A comprehensive examination of laboratory outcomes for children infected with influenza A and B revealed notable differences in lymphocyte and platelet counts across all age groups. Children with influenza A/H1N1 had significantly higher lymphocyte levels than those with influenza A/H3N2 or B. Additionally, in children aged 3 and older, those with influenza A/H1N1 exhibited higher platelet counts than those with influenza B. Similar findings have been reported in other studies (8). This observation underscores that infections caused by influenza A and B exhibit discernible differences across various facets, including pathogenic mechanisms, the magnitude of the host's immune response, and the degree of organ damage inflicted (14, 15). While co-infections involving influenza viruses and other respiratory pathogens are frequently reported, detailed analyses comparing co-infection rates across various influenza virus types and subtypes remain uncommon.
Influenza A/H1N1 has a co-infection rate of 3.5%, significantly lower than the 11.9% for influenza A/H3N2 and 10.6% for influenza B, highlighting notable differences in co-infection rates among these influenza types. Studies have shown that infections and immune responses caused by influenza viruses can alter the composition and function of respiratory microbiota. These changes, in turn, may modify the immune response to subsequent secondary pathogen infections or alter the dynamics of microbial interactions, thereby enhancing or inhibiting the proliferation of other potential pathogenic microorganisms (5, 16). This may explain the variation in co-infection rates among different types of influenza viruses. Other respiratory viruses remain the most common co-pathogens with all three influenza viruses. Among the co-pathogens of influenza A/H1N1, A/H3N2, and influenza B, the proportions of viral co-infections are 100%, 64.2%, and 76.3%, respectively. This observation aligns with previous studies that have documented the co-circulation and co-infection dynamics of influenza viruses with other respiratory viruses (17, 18). Additionally, the risk of co-infection increases when the epidemic periods of different pathogens overlap. In our study, the simultaneous outbreaks of Influenza A/H3N2 and M. pneumoniae may explain the elevated co-infection rate of 24.7%.
The impact of co-infection on illness severity or outcomes is still being debated (7, 19). We found that children infected with influenza B or influenza A/H3N2 who were co-infected with other respiratory pathogens exhibited differences in clinical manifestations, pneumonia incidence, and hospital stay duration compared to those with single infections. Importantly, the findings reveal that M. pneumoniae is a common co-pathogen in influenza A/H3N2 cases, occurring in 24.7% of instances. This supports previous reports suggesting that M. pneumoniae may contribute to greater disease severity and poorer clinical outcomes in cases of influenza pneumonia (20). Consequently, we speculate that co-infection with other respiratory pathogens may exacerbate the condition in children infected with these two viruses. In contrast, no such differences were observed in the case of single and co-infections with influenza A/H1N1, consistent with previous reports (14).
The findings of this retrospective analysis should be interpreted with caution due to several limitations. Firstly, this study is based on data from a single center, which may limit the generalizability of the results to a broader population. Additionally, the relatively small number of co-infection cases involving influenza A/H1N1 could introduce bias in the statistical outcomes, so the conclusions drawn from these findings should be approached with careful consideration. Moreover, the significant prevalence of antibiotic use prior to admission in more than half of the children is a factor that could potentially affect the rates of co-infections, particularly bacterial co-infections. Further research involving larger, more diverse cohorts would be beneficial to provide deeper insights and enhance understanding of these complex interactions.
5.1. Conclusions
Our study highlights the influence of different influenza virus types/subtypes on the clinical manifestations, pneumonia incidence, and hospitalization duration in pediatric infections. In children aged six and older, influenza A/H3N2 infections are associated with a higher rate of pneumonia and longer hospital stays. Co-infections with other respiratory pathogens worsen the condition of patients with influenza B or A/H3N2.