1. Background
World populations are infected with herpes simplex virus (HSV) at an alarming rate (1). Herpes simplex virus is a well-established cause of eye disease and associated blindness. Herpes simplex keratitis (HSK) is a condition caused by the invasion of HSV into the cornea (2). It manifests as a variety of symptoms such as pain and dryness, lacrimation, and impaired vision. Herpes simplex keratitis is difficult to treat, with poor prognosis, leading to severe negative effects on the quality of life of patients (3). Various antiviral drugs are used locally to treat HSK, of which acyclovir eye drops and ganciclovir gel are the most commonly prescribed, while antibiotics may also be used (4). A recent study (5) pointed out that ganciclovir gel is a rapid and safe method in treating HSK. A particularly beneficial effect of its use in HSK is that it is particularly effective at reducing the neuropathic pain sequelae caused by viral invasion if applied in the early stages. However, it also has certain limitations, including inefficacy in improving immunity and alleviating pain (6). Potassium sodium dehydroandroandrographolide succinate (PSDS), an extract from the herba andrographitis (Andrographis paniculata), is mainly used to treat viral pneumonia and upper respiratory tract infections as it confers antiviral, anti-inflammatory, and analgesic effects (7).
2. Objectives
We undertook this preliminary clinical study to determine whether PSDS offers therapeutic benefits in HSK patients treated with ganciclovir and to evaluate its effects on ocular and systemic inflammation and oxidative stress during the treatment period.
3. Methods
3.1. Research Subjects
We performed a preliminary clinical study to evaluate the efficacy of PSDS in combination with ganciclovir in patients with HSK. A total of 80 patients with HSK, diagnosed by clinical manifestations and previous medical records, who were admitted to The People’s Hospital of Leshan from April 2017 to January 2020, were enrolled in this study. Before enrollment, the patients signed informed consent, and study approval was obtained from the Ethics Committee of The People’s Hospital of Leshan.
Inclusion criteria were set as follows: (1) Patients with a confirmed diagnosis of HSK (HSK is diagnosed by examining corneal scrapings to detect HSV DNA in collected tear fluid using real-time polymerase chain reaction) (8); (2) patients aged 40 - 60 years; and (3) those requiring a hospital stay of over 10 days. Exclusion criteria were: (1) Patients with mental illness; (2) patients with malignant tumors; (3) patients with a previous history of eye surgery; (4) patients with systemic inflammation or corneal perforation; (5) patients whose treatment plan was adjusted due to various emergency situations during hospitalization; or (6) patients allergic to the drugs used in this study or undergoing other drug and/or physical therapies within 1 month before enrollment.
All 80 patients who met the inclusion criteria were completely randomized into the ganciclovir + PSDS group (40 cases) and the control group (40 cases) under double-blind conditions using a randomized table method in a 1:1 ratio. There were 21 males and 19 females aged 40 - 60 years, with an average age of (53.2 ± 2.4 years) in the ganciclovir + PSDS group. In terms of lesion location, there were 19 cases on the right, 19 cases on the left, and 2 cases on both sides. The course of the disease ranged from 3 - 9 days, with an average of 5.1 ± 1.1 days. Regarding corneal lesions, 15 cases were geographic, 12 cases were dendritic, and 13 cases were discal.
In the control group, there were 20 males and 20 females aged 40 - 60 years, with an average age of 53.3 ± 2.5 years. There were 20 cases on the right, 19 cases on the left, and 1 case on both sides. The course of the disease ranged from 3 - 8 days, with a mean of 5.0 ± 1.0 days. Additionally, 16 cases were geographic, 13 cases were dendritic, and 11 cases were discal. No statistically significant differences were found in gender, age, lesion location, disease course, and corneal lesions between the two groups (P > 0.05).
3.2. Treatment Procedure
For HSK patients with obvious pain, attention was paid to maintaining their nutritional function, pregabalin was used early to prevent neuropathic pain, and neurotrophic support and other treatments were strengthened simultaneously. In the control group, ganciclovir gel (Hangzhou Zhongmei Huadong Pharmaceutical, NMPN H20057690, batch number: 2017 BH 0305) was applied 5 - 6 times a day, 2 drops each time. The patients in the ganciclovir + PSDS group were additionally intravenously infused with PSDS (400 mg added into 5% glucose injection, Zhuhai Biochemical Plant, NMPN: H20045393, batch number: 20170326) once a day, in addition to ganciclovir gel. Each course of treatment lasted 10 consecutive days.
3.3. Ganciclovir + Dehydroandroandrographolide Succinate Indexes
The concentrations of serum C5 and inflammatory and oxidative stress factors in the serum and tear fluid at 10 days after treatment were compared between the two groups. The correlations between changes in serum C5 concentration and changes in the concentrations of high-sensitivity C-reactive protein (hs-CRP) and the oxidative stress factor superoxide dismutase (SOD) in the serum and tear fluid were also analyzed. The time to improvement of clinical symptoms (dryness, eye pain, corneal wound healing, and lacrimation) was recorded in both groups. The results of four examinations for tear film in the tear film stability test were compared between the two groups. Additionally, the efficacy outcomes were recorded in both groups.
3.4. Evaluation Criteria
The concentration of serum C5 was measured via double-antibody sandwich enzyme-linked immunosorbent assay. The substrate TMB was used for color development, and the optical density was read using a microplate reader at a wavelength of 450 nm. The serum C5 kit was provided by Xiamen Huijia Biotechnology Co., Ltd. All operations were strictly performed by laboratory technicians with more than 10 years of work experience in accordance with the instructions. High-sensitivity C-reactive protein and tumor necrosis factor-α (TNF-α) and the oxidative stress factors malondialdehyde (MDA) and SOD were measured in serum and tear fluid. For the tear film stability test, four standard measures were assessed in line with the tear film and ocular surface society (TFOS) dry eye workshop (DEWS) II diagnostic methodology subcommittee recommendations. Measures were: (1) Tear film break-up time (BUT) using the tear film stability test with fluorescein dye under topical anesthesia observed under cobalt blue light; (2) ocular surface damage assessment score by corneal staining with fluorescein dye; (3) volume of tear fluid secreted using the Schirmer I test; and (4) tear meniscus height (TMH) assessment using a cobalt blue filter (9-12).
The overall response to treatments was classified into remission (with completely absent clinical symptoms and signs: Absence of pain; absence of herpes lesions; and a normal corneal fluorescence staining result), improvement (with basically relieved clinical symptoms and signs, a Pain Numeric Rating Scale (NRS) score less than 3 points, no new herpes lesions, and weekly positive corneal fluorescence staining after treatment), and Ineffectiveness (with unimproved or worsened clinical symptoms and signs, and a new herpes outbreak after treatment).
3.5. Statistical Analysis
Measurement data were expressed as mean ± standard deviation (
4. Results
4.1. Comparison of Serum C5 Concentration During Treatment Between the Two Groups
Before treatment, there was no statistically significant difference in the serum C5 concentration between the two groups. At 3, 7, and 10 days after treatment, serum C5 concentration in the ganciclovir + PSDS group was significantly lower than that in the control group (P < 0.005) (Table 1).
| Variables | Before Treatment | At 3 Days After Treatment | At 1 Week After Treatment | At 10 Days After Treatment |
|---|---|---|---|---|
| G + PSDS group | 356.5 ± 19.8 | 186.9 ± 8.9 | 154.7 ± 7.3 | 136.3 ± 6.5 |
| Control group | 356.6 ± 20.0 | 285.8 ± 15.6 | 265.6 ± 10.3 | 219.8 ± 5.5 |
| t | 0.023 | 34.827 | 55.558 | 62.023 |
| P-value | 0.982 | < 0.005 | < 0.005 | < 0.005 |
Comparison of Change Trend of Serum C5 Concentration During Treatment Between the Two Groups (ng/mL,
4.2. Comparisons of Concentrations of Serum Inflammatory and Oxidative Stress Markers at 10 Days After Treatment Between the Two Groups
At 10 days after treatment, the concentrations of serum hs-CRP, TNF-α, MDA, and SOD were lower in the ganciclovir + PSDS group than in the control group (P < 0.005) (Table 2).
| Variables | hs-CRP (mg/L) | TNF-α (g/L) | MDA (mol/L) | SOD (U/L) |
|---|---|---|---|---|
| G + PSDS | 6.9 ± 0.5 | 50.4 ± 5.1 | 3.2 ± 0.2 | 0.8 ± 0.1 |
| Control group | 12.5 ± 1.4 | 135.6 ± 10.2 | 6.6 ± 0.4 | 0.4 ± 0.1 |
| t | 23.824 | 47.251 | 48.083 | 17.889 |
| P-value | < 0.005 | < 0.005 | < 0.005 | < 0.005 |
Comparisons of Concentrations of Serum Inflammatory and Oxidative Stress Markers at 10 Days After Treatment Between the Two Groups (
4.3. Comparisons of Concentrations of Inflammatory and Oxidative Stress Factors in Tears at 10 Days After Treatment Between the Two Groups
Compared with those in the control group, the concentrations of hs-CRP, TNF-α, MDA, and SOD in the tear fluid declined in the ganciclovir + PSDS group at 10 days after treatment (P < 0.05) (Table 3).
| Variables | hs-CRP (mg/L) | TNF-α (g/L) | MDA (mol/L) | SOD (U/L) |
|---|---|---|---|---|
| G + PSDS | 8.6 ± 1.1 | 41.2 ± 4.3 | 2.6 ± 0.3 | 1.1 ± 0.2 |
| Control group | 15.9 ± 2.3 | 111.2 ± 11.8 | 7.8 ± 0.8 | 0.3 ± 0.1 |
| t | 18.109 | 35.251 | 38.492 | 22.627 |
| P-value | < 0.005 | < 0.005 | < 0.005 | < 0.005 |
Comparisons of Concentrations of Inflammatory and Oxidative Stress Markers in Tear Fluid at 10 Days After Treatment Between the Two Groups (
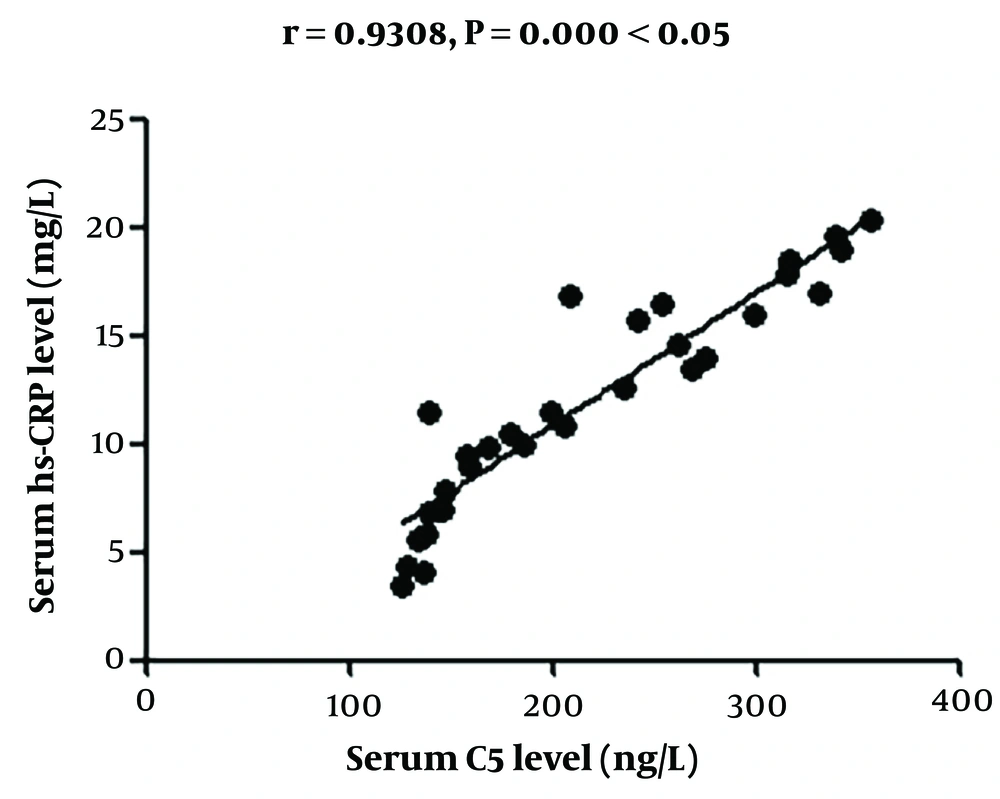
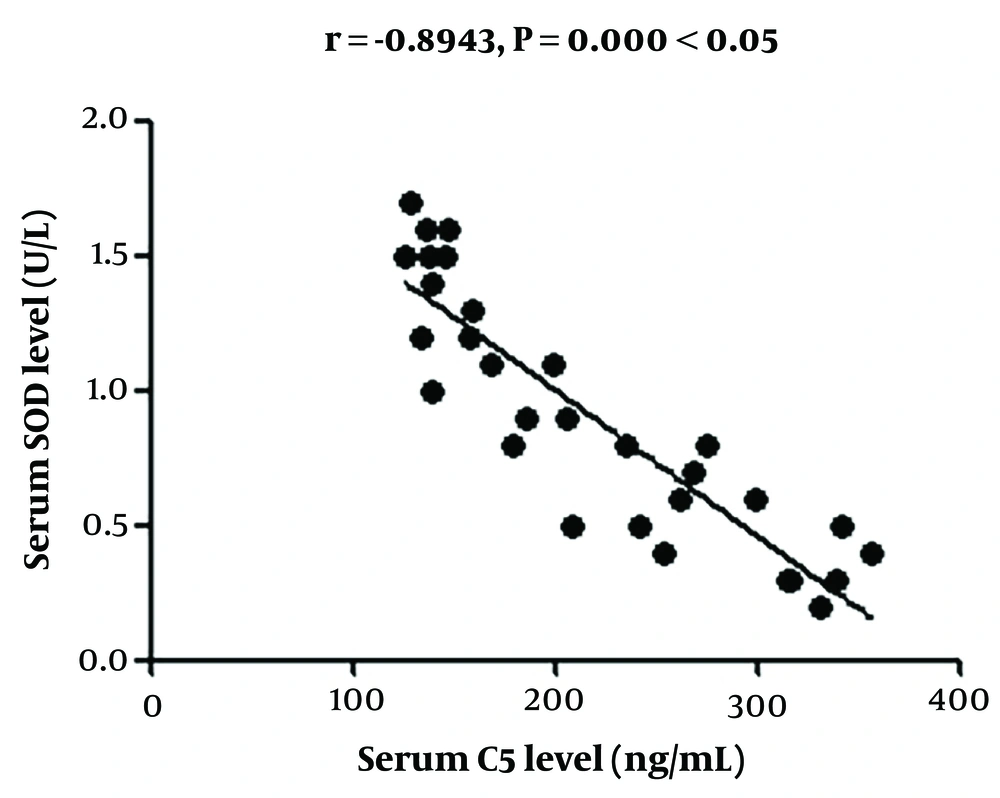
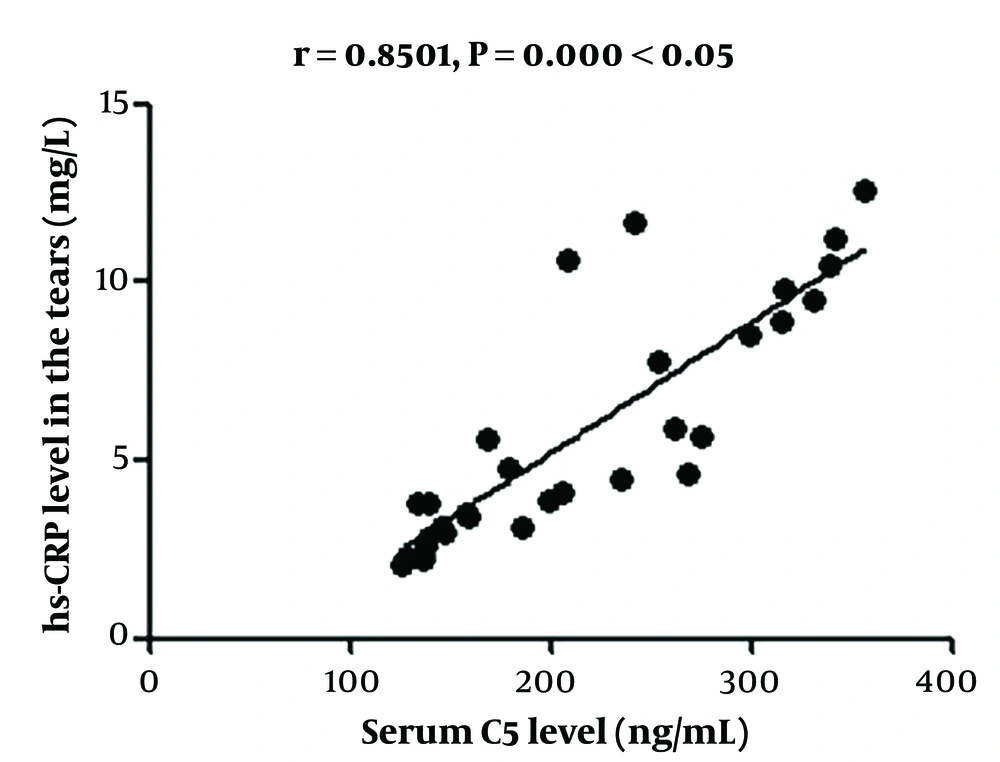
4.4. Correlation Analysis of Changes in Serum C5 Concentration with Changes in High-Sensitivity C-reactive Protein and Superoxide Dismutase Concentrations in Serum and Tear Fluid
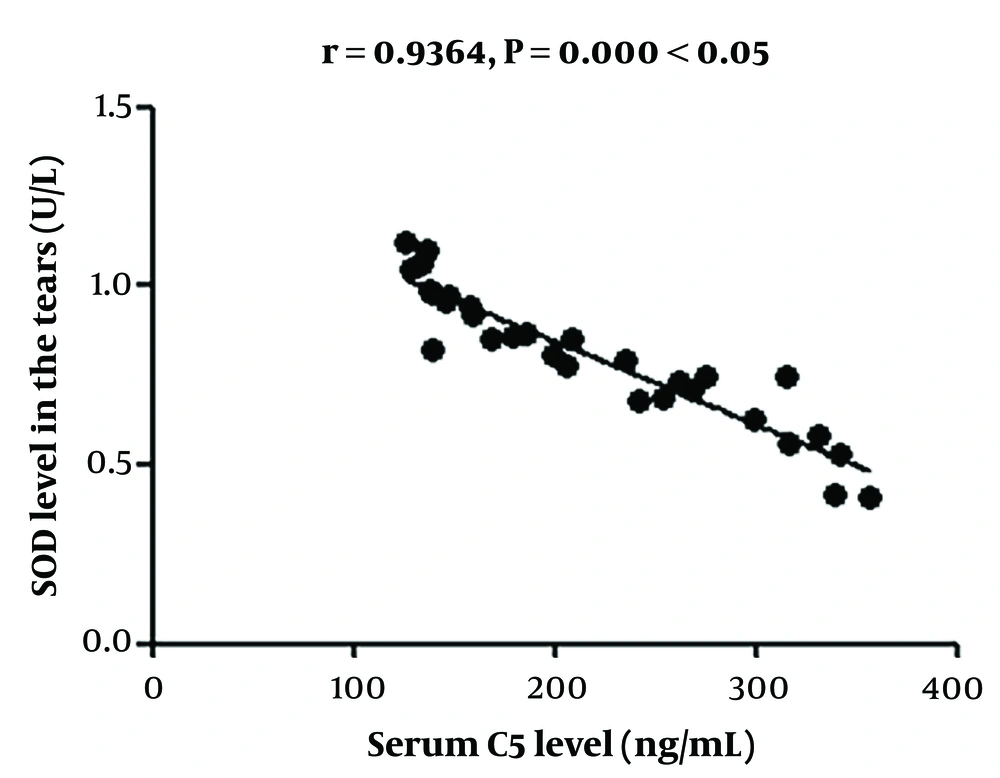
The change in serum C5 concentration had a positive correlation with that in hs-CRP concentration and a negative association with that in SOD concentration in the serum and tears (Figures 1 - 4).
4.5. Comparison of Time of Clinical Symptom Improvement Between the Two Groups
The time to improvement of the clinical symptoms (eye dryness, pain, corneal wound healing, and lacrimation) in the ganciclovir + PSDS group was significantly shorter than that in the control group (P < 0.005) (Table 4).
| Variables | Dryness in the Eyes | Pain in the Eyes | Corneal Wound Healing | Lacrimation |
|---|---|---|---|---|
| Ganciclovir + PSDS group | 3.8 ± 0.3 | 4.6 ± 0.6 | 8.9 ± 1.2 | 6.6 ± 0.8 |
| Control group | 5.8 ± 0.7 | 6.5 ± 1.1 | 10.3 ± 1.8 | 8.8 ± 1.3 |
| t | 16.609 | 9.590 | 4.093 | 9.115 |
| P-value | < 0.005 | < 0.005 | < 0.005 | < 0.005 |
Comparison of Time of Clinical Symptom Improvement Between the Two Groups (d,
4.6. Comparisons of Results of Four Examinations for Tear Film in Tear Film Stability Test Between the Two Groups
In comparison with the control group, the ganciclovir + PSDS group displayed a longer BUT (P < 0.005), a higher corneal fluorescence staining score (P < 0.005), a larger volume of tear secreted in the Schirmer I test, and a higher TMH (P < 0.005) (Table 5).
| Variables | Tear Film BUT (s) | Corneal Fluorescence Staining Score (Points) | Volume of Tear Secreted in Schirmer I Test (mm) | TMH (μm) |
|---|---|---|---|---|
| Ganciclovir + PSDS group | 10.3 ± 0.8 | 29.8 ± 0.6 | 11.1 ± 0.3 | 0.6 ± 0.1 |
| Control group | 5.1 ± 0.2 | 38.1 ± 2.5 | 8.1 ± 0.1 | 0.4 ± 0.1 |
| t | 39.882 | 20.418 | 60.000 | 8.944 |
| P-value | < 0.005 | < 0.005 | < 0.005 | < 0.005 |
Comparisons of Results of Four Examinations for Tear Film in Tear Film Stability Test Between the Two Groups (
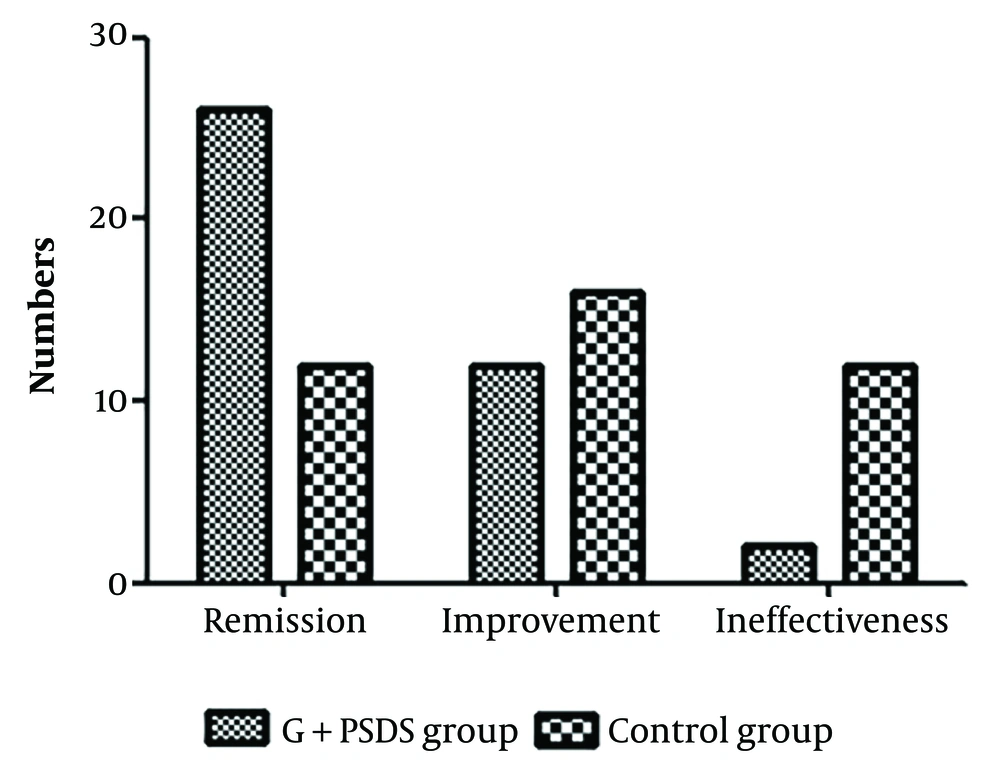
4.7. Comparison of Clinical Efficacy Between the Two Groups
There were 26 cases of Remission, 12 cases of Improvement, and 2 cases of Ineffectiveness in the ganciclovir + PSDS group. In the control group, there were 12 cases of Remission, 16 cases of Improvement, and 12 cases of Ineffectiveness. The remission rate was higher in the ganciclovir + PSDS group than in the control group (χ² = 9.825, P = 0.002), and the ineffectiveness rate was lower in the ganciclovir + PSDS group than in the control group (χ² = 7.013, P = 0.008) (Figure 5).
5. Discussion
Herpes simplex keratitis is a relatively common infectious eye disease in the clinic, mainly caused by HSV infection (13). It ranks first in incidence rate among corneal diseases and may lead to neuropathic pain sequelae, with blindness as a potential end-stage outcome of the disease (14). Each year, approximately 1.8 million people worldwide contract HSV keratitis, including more than 40,000 new cases that result in severe visual impairment (15). In addition, it has a high relapse rate after treatment and a long clinical course. For this reason, pragmatic and effective early intervention is of great significance for ensuring the effectiveness of clinical treatment, shortening the clinical course, and reducing the incidence of stromal scarring, astigmatism, neovascularization, ulceration, and perforation (16).
For patients with an initial outbreak, antiviral treatment is primarily adopted. Ganciclovir, a new type of 2'-deoxyguanine nucleotide drug, is capable of quickly forming triphosphate compounds and specifically binding to giant cells in the body to suppress virus growth (17). In addition, it can repress the replication of viral DNA by competitively inhibiting herpes-zoster virus DNA polymerase and reducing the binding of herpes-zoster virus DNA to nerve cells (18, 19). Ganciclovir has certain value for treating new cases, but ganciclovir treatment alone is associated with frequent recurrence after treatment and even post-stage neuropathic pain complications (20).
A study (21) found that C5, an inflammation-related factor, has immune regulatory functions, with an overtly raised expression in many immune diseases, including the HSV immune response. C5 is a chemokine for various inflammatory cells and can effectively promote the fusion of lysosomes and cell membranes, playing an important role in the body's immune response. Moreover, it can also induce the production of various inflammatory cytokines, including TNF-α and interleukin-1, activate the NF-κB signaling pathway, and enhance the aseptic inflammatory response, thereby aggravating damage to tissues and cells (22). In this study, ganciclovir gel was adopted in the control group, while PSDS combined with ganciclovir gel was used in the ganciclovir + PSDS group to treat HSK. Serum C5 concentration in the ganciclovir + PSDS group was markedly lower than that in the control group at 3, 7, and 10 days after treatment, suggesting that PSDS combined with ganciclovir is able to effectively decrease serum C5 concentration in HSK.
In addition, the concentrations of hs-CRP, TNF-α, MDA, and SOD in both serum and tear fluid were lower in the ganciclovir + PSDS group compared to the control group after treatment, implying that PSDS combined with ganciclovir can effectively reduce both inflammatory responses and oxidative stress in HSK patients. Furthermore, the change in C5 concentration had a positive correlation with the change in hs-CRP concentration and a negative association with the change in SOD concentration in the serum and tears, indicating that changes in C5 concentration are robustly associated with changes in the inflammatory and oxidative stress markers. Furthermore, the time to clinical symptom improvement was compared between the two groups, and it was found that the time to improvement of eye dryness and pain, corneal wound healing, and lacrimation in the ganciclovir + PSDS group was considerably shorter than that in the control group. This suggests that PSDS combined with ganciclovir is capable of effectively mitigating the clinical symptoms of patients.
Subsequently, in comparison with the control group, the ganciclovir + PSDS group displayed a longer tear film BUT, a higher corneal fluorescence staining score, a larger volume of tears secreted, and a higher TMH, implying that PSDS combined with ganciclovir in the treatment of HSK can effectively improve tear properties. Lastly, the clinical efficacy was found to be superior in the ganciclovir + PSDS group. The remission rate was higher in the ganciclovir + PSDS group than in the control group, and the ineffectiveness rate was lower in the ganciclovir + PSDS group than in the control group. These findings further indicate that, for HSK, PSDS combined with ganciclovir may provide considerable improvements in clinical efficacy.
Ganciclovir alone, applied for the treatment of HSK in this study, is capable of effectively suppressing viral infection, with high safety and fast onset. However, it had no obvious effects on improving inflammation or oxidative stress or in reducing relapse. As an extract of potassium sodium salt from andrographolide succinic acid, PSDS, used in the ganciclovir + PSDS group, can effectively enhance the resistance and immunity of the body (23). Additionally, it can lower capillary permeability, inhibit inflammatory responses, reduce tissue edema, and improve the activity of peripheral neutrophils and macrophages to some extent (24).
In vitro tests (25) have shown that PSDS can effectively kill multiple viruses, such as adenovirus, HSV, respiratory syncytial virus, and rhinovirus, and also has a certain inhibitory effect on various pathogenic bacteria (26). Potassium sodium dehydroandroandrographolide succinate combined with ganciclovir gel in treating HSK significantly cleared the virus and reduced inflammation and oxidative stress (27). The strengths of our study are the detailed assessment of clinical outcomes and the mechanistic evaluation of inflammatory and oxidative stressors, including the novel finding of C5 as a marker of the HSK course. This is the first study to report the potential efficacy of ganciclovir in combination with PSDS for the treatment of HSK.
However, this was a preliminary clinical study, which entails several limitations. First, the doses of ganciclovir gel were mismatched between the groups, with the ganciclovir + PSDS group receiving a lower dose during the 10-day treatment period. Second, the results of the study were limited to a 10-day treatment period, and certain clinical outcomes were subjective and variable, potentially affecting the assessment of efficacy. Future studies need to use standardized and objective measures to assess efficacy over longer follow-up periods and account for potential long-term side effects. Third, an initial sample size calculation was not performed. However, post hoc power analyses indicated that in independent samples t-tests and χ² analyses with 40 individuals per group, we had 80% power to detect a moderate effect size of 0.6 (Cohen’s d).
Fourth, because the study focused on potential anti-inflammatory mechanisms of action, viral load was not tested, and the impact of treatment on antivirals could not be assessed. We hope that the current preliminary results will stimulate interest in initiating and designing larger clinical trials to determine the efficacy of PSDS for HSK treatment. In addition, although biochemical indices in serum and tears provide new insights into assessing the clinical efficacy of PSDS in HSK, they still need to be supported by a large number of high-quality clinical and laboratory studies to confirm their validity and utility as surrogate endpoints. Future studies should aim to establish a strong link between these indices and clinical outcomes of PSDS for HSK.
5.1. Conclusions
In conclusion, the serum C5 concentration of HSK patients was significantly elevated and was closely related to inflammation and oxidative stress factors. The application of PSDS combined with ganciclovir effectively reduced the concentration of C5, inhibited the inflammatory response, enhanced the body’s antioxidant capacity, and significantly improved the clinical symptoms of the patients, with better efficacy than ganciclovir alone.