1. Background
The rising concern over antibiotic resistance has created a substantial threat to human health (1). Consequently; there has been a recent surge of scholarly interest in antimicrobial peptides (AMPs) as potential alternatives to traditional antibiotics. Antimicrobial peptides have demonstrated remarkable efficacy against bacteria; viruses; and fungi; while also reducing inflammation and enhancing the innate immune system; positioning them as a promising next generation of antibiotics (2). The primary mechanism of action of AMPs generally involves disrupting microbial membranes; leading to cell lysis or penetration of lipid membranes; followed by targeting intracellular components (3). Among known AMPs; two peptides; Ib-AMP4 and Oncorhyncin II; have shown significant potential. Ib-AMP4; an antimicrobial peptide derived from the seeds of Impatiens balsamina; functions as part of the plant’s defense system and carries a net charge of +6 (4).
In contrast; Oncorhyncin II is a low-toxicity AMP composed of 69 amino acids; sourced from the skin of rainbow trout (5). Both peptides have demonstrated strong antimicrobial effects against both Gram-positive and Gram-negative bacteria; as reported in previous research (2). Acinetobacter baumannii; a gram-negative bacterial strain; poses a considerable challenge to public health due to its resistance to various antibiotics; including penicillin. This resistance has become a critical factor in the spread of hospital-acquired infections; including bloodstream infections; soft tissue and skin infections; ventilator-associated pneumonia; and infections related to urinary catheters (6).
2. Objectives
Given the high incidence of A. baumannii infections and the urgent need for innovative treatments, along with the extensive research on antimicrobial peptides, this study aims to produce recombinant Ib-AMP4 and Oncorhyncin II peptides and explore their effectiveness in combating A. baumannii (ATCC 19606). Additionally, this research seeks to provide valuable insights into potential new treatment options for A. baumannii infections. By achieving these goals, the study’s findings may contribute to the development of effective therapies against antibiotic-resistant pathogens, a critical concern in modern healthcare.
3. Methods
3.1. Procurement of Materials and Chemicals
The materials and chemicals used in this investigation were obtained from reputable suppliers as follows: Ampicillin, chloramphenicol, nutrient agar (NA), nutrient broth (NB), Mueller-Hinton agar (MHA), Mueller-Hinton broth (MHB), and resazurin sodium salt were sourced from Sigma-Aldrich, USA. The Ni-NTA kit was procured from QIAGEN, USA, and Polymyxin E was purchased from Sigma-Aldrich.
3.2. Genetic Elements, Vectors, and Bacterial Hosts
In this investigation, we used the plasmids pET32a-Ib-AMP4 and pET32a-Oncorhyncin II, which were obtained from prior research conducted by Abtahi. The Escherichia coli BL21 (DE3) pLysS strain (Novagen, USA) was selected as the host strain for expressing the recombinant proteins. Antibacterial activity tests were performed against the A. baumannii (ATCC 19606) microorganism.
3.3. Expression of Ib-AMP4 and Oncorhyncin II
The pET32a-Ib-AMP4 and pET32a-Oncorhyncin II plasmids were transferred into E. coliBL21 (DE3) pLysS to express the target proteins. The protein expression process involved the following steps:
(1) A single colony of transformed E. coliBL21 (DE3) pLysS was selected from petri dishes containing ampicillin and chloramphenicol. This colony was then cultured in 2 mL of nutrient broth (NB) medium with antibiotics at concentrations of 100 mg/L for ampicillin and 34 mg/L for chloramphenicol.
(2) Subsequently, 0.5 mL of the overnight cultures were introduced into fresh NB medium containing the same antibiotic concentrations (100 mg/L of ampicillin and 34 mg/L of chloramphenicol) and incubated at 37°C with agitation.
(3) When the optical density at 600 nm reached 0.5 - 0.8, isopropyl β-D-1-thiogalactopyranoside (IPTG) was added at a concentration of 0.5 mM to induce protein expression.
(4) After a 4-hour induction period, the bacterial cells were harvested by centrifugation at 6000 rpm for 10 minutes at 25°C. The resulting pellets were stored at -20°C.
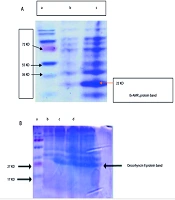
(5) Using these techniques, we aimed to investigate the antibacterial efficacy of Ib-AMP4 and Oncorhyncin II against A. baumannii (ATCC 19606) while evaluating the quantity of expressed proteins using SDS-PAGE (12%) (refer to Figure 1).
3.4. Purification of Ib-AMP4 and Oncorhyncin II
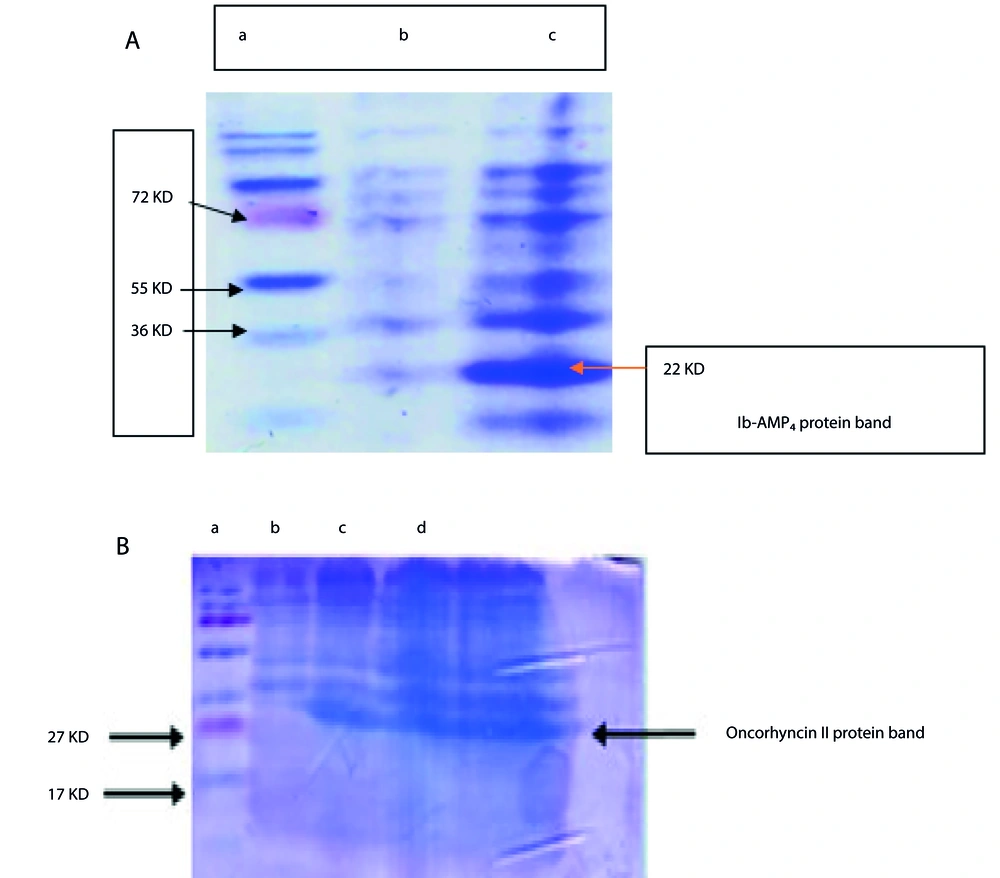
We purified the Ib-AMP4 and Oncorhyncin II proteins using Ni-NTA affinity chromatography according to the manufacturer’s instructions. To assess the recombinant proteins, we conducted sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel (see Figure 2). Additionally, the quantification of the purified proteins was determined by calculating the absorbance ratio at 260 nm and 280 nm (A260/A280) using the formula (1):
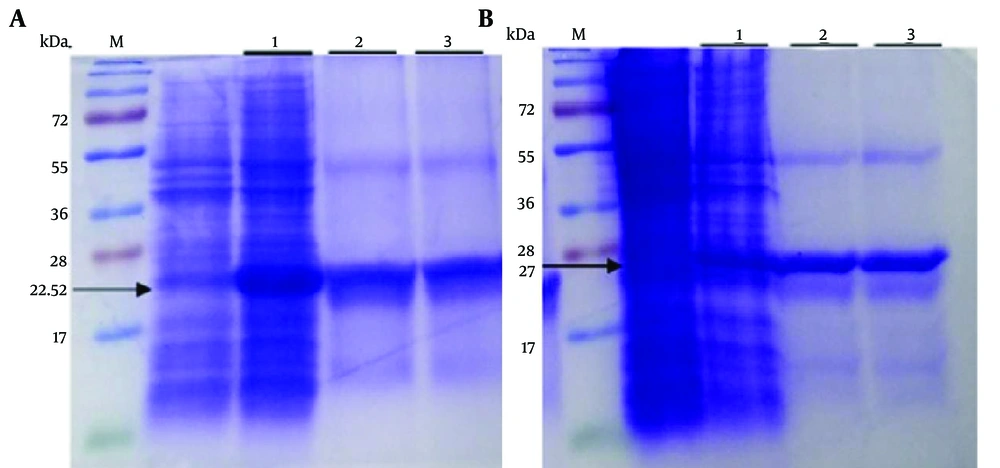
A, M: Size marker; lane 1: Expression of Ib-AMP4 in Escherichia coli BL21 (DE3) pLysS; lane 2: Purified protein retrieved by Nickel affinity chromatography; lane 3: Ib-AMP4 protein after refolding. B, M: Size marker; lane 1: Expression of Oncorhyncin II in E.coli BL21 (DE3) pLysS; lane 2: Purified protein retrieved by Nickel affinity chromatography; lane 3: Oncorhyncin II protein after refolding
The primary aim of these purification procedures was to examine the antibacterial activity of Ib-AMP4 and Oncorhyncin II against A. baumannii (ATCC 19606).
3.5. Urea Detection Test
To verify the absence of urea in the protein solution, we performed a urea assay using the Berthelot kit (Iran), strictly adhering to the protocol provided by the manufacturer. This step was essential to ensure the purity of the protein solution for subsequent analyses.
3.6. Improving Refolding of Ib-AMP4 and Oncorhyncin II
To ensure the successful refolding of the peptides and prevent any adverse effects on their active configuration, eliminating urea was essential. We employed a dialysis method using phosphate-buffered saline (PBS)-based exchange buffers with specific amino acid compositions: 0.1 M arginine and 0.1 M proline at an optimal pH of 7.2 for Ib-AMP4, and 0.01 M arginine and 0.1 M proline at an optimal pH of 8.5 for Oncorhyncin II (7, 8). During the 24-hour dialysis process, the temperature was maintained at 4°C to preserve stability. The PBS solution was refreshed every 2 hours to enhance efficiency. Following dialysis, the protein samples were stored at 4°C for further analysis (9). To confirm the purity of the recombinant proteins, we conducted SDS-PAGE on a 12% gel (refer to Figure 2). Additionally, the purified protein quantities were calculated by determining the absorbance ratio at 260 nm and 280 nm (A260/A280) using the specified formula (1).
3.7. Concentration of Ib-AMP4 and Oncorhyncin II
To concentrate the recombinant proteins Ib-AMP4 and Oncorhyncin II, we used a 10-kDa pore-size Amicon centrifugal filter. The proteins were concentrated into dialysis tubes supplied by Merck Millipore, Darmstadt, Germany.
3.8. Antimicrobial Activity of Ib-AMP4 and Oncorhyncin II
3.8.1. Evaluation of Minimum Inhibitory Concentration
According to CLSI MO7-A10 guidelines (10), the broth microdilution method was employed to determine the minimum inhibitory concentration (MIC) of Ib-AMP4 and Oncorhyncin II. Initially, A. baumannii cultures were diluted overnight in fresh MH broth (Mueller Hinton Broth; Merck, Germany) until the optical density (OD) reached 1 × 108 CFU/mL. This culture was further diluted to 1 × 106 CFU/mL in MH broth. Then, 50 μL of the bacterial dilutions (1 × 106 CFU/mL) were mixed with 50 μL of the dialyzed recombinant proteins Ib-AMP4 (222 µg/mL) and Oncorhyncin II (321 µg/mL) in separate wells of 96-well plates. For the positive control, 100 μL of bacterial suspension was used, while the negative control contained 100 μL of sterilized MH broth.
The plates were incubated at 37°C for 24 hours. After incubation, 20 µL of a 0.02% w/v resazurin solution was added to each well and allowed to stand for 2 hours. The MIC was identified by observing the last well with a blue color, indicating inhibition of bacterial growth (11). Additionally, the MIC of Polymyxin E against A. baumannii was evaluated using the micro broth dilution method, following CLSI guidelines (12).
3.8.2. Evaluation of Minimum Bactericidal Concentration for Ib-AMP4 and Oncorhyncin II
In accordance with the established MIC values, we determined the minimum bactericidal concentration (MBC) for Ib-AMP4 and Oncorhyncin II using 100 μL microplate wells. The MBC represents the minimum concentration of the antimicrobial agents required to eliminate 99.9% of the target organism after a 24-hour incubation period at 37°C. To evaluate the antibacterial efficacy, we calculated the MBC/MIC ratio, where an MBC/MIC ratio of 1 or 2 indicates a bactericidal effect, and an MBC/MIC ratio of 4 or higher suggests a bacteriostatic effect (13).
3.8.3. Assessment of Synergistic Activities Between Ib-AMP4 and Oncorhyncin II
We conducted checkerboard assays using 96-well plates following CLSI guidelines to investigate potential synergistic effects between Ib-AMP4 and Oncorhyncin II. In these assays, Ib-AMP4 and Oncorhyncin II concentrations were incrementally diluted to determine their MICs and combined MICs. Oncorhyncin II concentrations were added along the vertical wells, while Ib-AMP4 concentrations were added across the horizontal wells. Bacterial colonies at a concentration of 1 × 106 CFU/mL were introduced into the wells. The recombinant peptides were then serially diluted and incubated at 37°C for 18 - 24 hours (14).
The checkerboard test evaluated the synergistic effect of the two recombinant proteins against A. baumannii ATCC 19606. The results indicated possible interactions, including synergistic, additive, antagonistic effects, or no interaction between the antimicrobial compounds. Typically, in vitro interactions of these compounds are evaluated based on the inhibitory concentration index; however, this study focused on the Fractional Inhibitory Concentration (FIC). The FIC for each antimicrobial agent, such as substance A, was calculated by dividing the MIC of substances A and B in combination by the MIC of substance A alone. The FIC index was calculated using the following formula:
The interpretation of the degree of synergy is as follows:
- FICI < 0.5 indicates a synergistic effect
- FICI between 0.5 and 0.75 suggests a relative synergy
- FICI between 0.76 and 1 indicates an additive effect
- FICI between 1 and 4 shows no interaction
- FICI > 4 suggests an antagonistic effect (14, 15).
Definitions:
- FIC = Fractional Inhibitory Concentration
- FICI = Fractional Inhibitory Concentration Index
3.8.4. Time-Kill Study
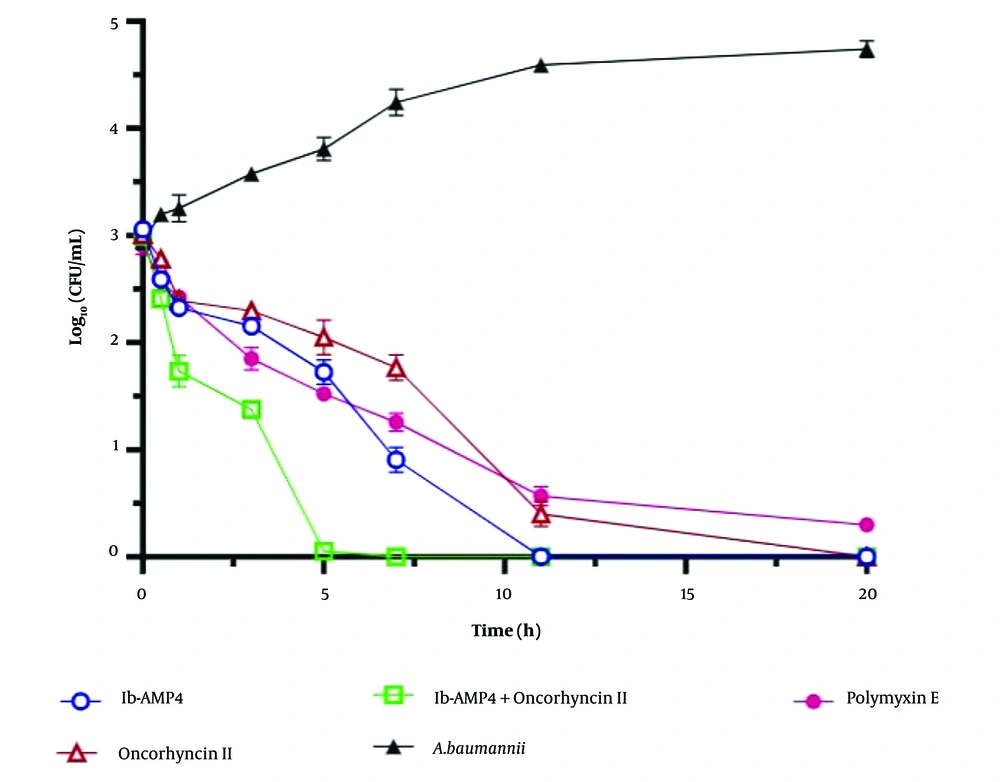
In this study, we employed a time-kill assay to evaluate the effectiveness of target recombinant antimicrobial proteins against A. baumannii. The initial bacterial concentration was set at 10^6 CFU/mL at the start of the experiment. We conducted tests on Ib-AMP4 and Oncorhyncin II individually and in combination at 2 × MIC concentrations to assess their impact on cell viability. At various time intervals (0, 0.5, 1, 3, 5, 7, 11, and 20 hours) after incubation, samples were collected and plated on MH agar to calculate colony counts.
The entire experiment was conducted in a 1 mL volume. After 24 hours of incubation at 37°C, we analyzed the data to evaluate synergistic effects and reduction patterns in viable bacterial cell counts. Polymyxin E (1 μg/mL) was used as a positive control, while a bacterial culture without any additions served as the negative control (refer to Figure 3). Antibacterial activity was defined as a reduction of ≥ 1 log₁₀ compared to the initial inoculum. A reduction of ≥ 2 log₁₀ indicated a synergistic effect, while a reduction of 1 ≤ log₁₀ ≤ 2 indicated an additive effect. Each test was replicated three times to ensure reliability (15).
3.8.5. Growth Kinetic Study
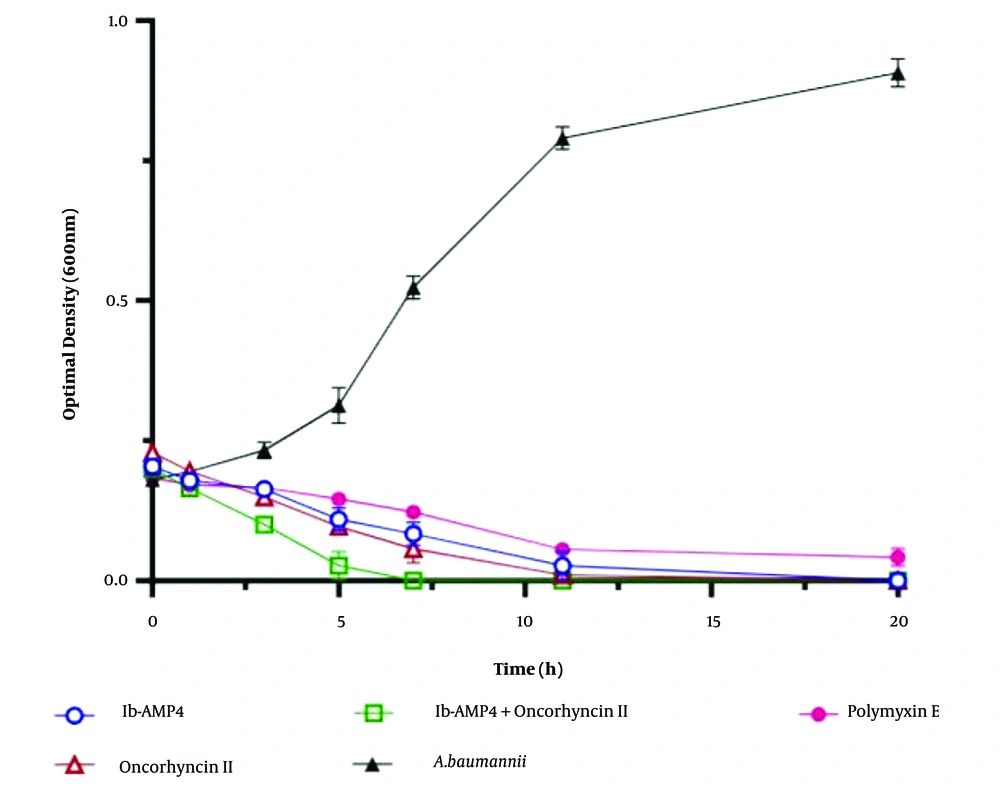
In this study, we aimed to assess the effectiveness of Oncorhyncin II against bacterial cultures. The bacterial cultures were allowed to reach the mid-log phase, achieving a turbidity of 0.6 at 600 nm. These cultures were then appropriately diluted in MHB to an OD600 of 0.2. Next, 200 μL of each diluted bacterial culture was transferred into individual vials. We then introduced 2 × MIC concentrations of Ib-AMP4 and Oncorhyncin II, individually and in combination, into designated vials. The vials were incubated at a constant temperature of 37°C. To monitor the experiment's progress, we measured the optical density of the samples at specific time intervals (0, 1, 3, 5, 7, 11, and 20 hours) using a spectrophotometer set to a wavelength of 600 nm. The entire experiment was conducted in triplicate to ensure accuracy.
For comparison, we used Polymyxin E at a concentration of 1 μg/mL as a positive control. Additionally, untreated bacteria served as the negative control (refer to Figure 4) (7). Through this methodology, we aimed to evaluate the efficacy of Oncorhyncin II against bacterial cultures and explore the potential for combination therapy in combating A. baumannii infections.
3.8.6. Data Analysis
To analyze the collected data, we conducted a two-way analysis of variance (ANOVA), with statistical significance determined by P-values below 0.05. All statistical analyses were performed using GraphPad Prism software, version 9.
4. Results
4.1. Ib-AMP4 and Oncorhyncin II Manifestation in Escherichia coli (DE3) pLysS
In this study, we successfully produced recombinant proteins, Ib-AMP4 (22.52 kDa) and Oncorhyncin II (27 kDa), using Escherichia coliBL21 (DE3) pLysS cells. Protein production was confirmed via SDS-PAGE analysis. Figure 1 displays the results, showing distinct bands that confirm the presence of the produced proteins.
4.2. Purification of Ib-AMP4 and Oncorhyncin II
The Ni-NTA kit was utilized to purify the recombinant proteins, which featured a 6× His-tag sequence at the C-terminal. The purity of the isolated proteins was assessed using SDS-PAGE, as shown in Figure 2.
4.3. Urea Assay Test
To ensure the accuracy of our in vitro experiments, it was essential to verify that the purified, refolded product was free of urea. Urea has antimicrobial properties that could interfere with our results. We used standard methods to confirm its absence, thus preventing any potential impact on our experimental findings.
4.4. Refolding of Ib-AMP4 and Oncorhyncin II
An MIC test was conducted to optimize dialysis conditions against A. baumannii. The ideal conditions were found to be at pH 7.2 for Ib-AMP4 and 8.5 for Oncorhyncin II, with the addition of 0.1 M arginine and 0.1 M proline. These conditions achieved maximum protein efficiency. However, the post-dialysis concentrations of Ib-AMP4 and Oncorhyncin II were initially insufficient for subsequent assays. To address this, Amicon centrifugal filters were used, significantly increasing the protein concentrations to 444 μg/mL for Ib-AMP4 and 642 μg/mL for Oncorhyncin II. In summary, the successful synthesis and refinement of Ib-AMP4 and Oncorhyncin II were achieved, and the optimized dialysis conditions allowed for a higher concentration of these proteins, rendering them suitable for further assays and investigations.
4.5. In Vitro Assays
4.5.1. Comparative Minimum Inhibitory Concentration of Engineered Ib-AMP4 and Oncorhyncin II Against Acinetobacter baumannii
To evaluate the efficacy of engineered Ib-AMP4 and Oncorhyncin II against A. baumannii, MIC values were determined. The MIC testing was conducted using a 96-well plate, with the initial well receiving the highest protein concentration, followed by serial halving of the protein concentration across wells 1 to 10. The MIC values obtained were 111 μg/mL for recombinant Ib-AMP4 and 80.25 μg/mL for Oncorhyncin II. The positive control used MHB, while the negative control contained a culture of A. baumannii (10). This analysis provided valuable insights into the potential effectiveness of the engineered peptides against A. baumannii, suggesting promising prospects for their utility in combating bacterial infections.
4.5.2. Measuring Minimum Bactericidal Concentration
The MBC findings indicated that both Ib-AMP4 and Oncorhyncin II peptides exhibited bacteriostatic properties. Table 1 provides the MBC values for each peptide individually as well as in combination.
| Microorganism | MBC (µg/mL) | MBC Combination (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ib-AMP4 | Oncorhyncin II | Ib-AMP4 | Oncorhyncin II | |||||
| Interpretation | Bactericidal | Bactericidal | Bactericidal | Bactericidal | ||||
| Acinetobacter baumannii | MBC | MBC/MIC | MBC | MBC/MIC | MBC | MBC/MIC | MBC | MBC/MIC |
| (ATCC 19606) | > 222 | > 2 | > 160.5 | > 2 | > 55.5 | > 2 | > 160.5 | > 2 |
Abbreviations: MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration.
4.5.3. Evaluation of Synergistic Effects Between Ib-AMP4 and Oncorhyncin II
Checkerboard assays were performed to confirm the synergistic effects of combining Ib-AMP4 with Oncorhyncin II. The results revealed a cooperative interaction between the two peptides against A. baumannii. The Fractional Inhibitory Concentration Index (FICI) values, shown in Table 2, detail the individual effects of each peptide as well as their combined impact.
| Microorganism | MIC (µg/mL) | MIC Combination (µg/mL) | Synergism | |||||
|---|---|---|---|---|---|---|---|---|
| Ib-AMP4 | FIC | Oncorhyncin II | FIC | Ib-AMP4 | Oncorhyncin II | FIC | ||
| Acinetobacter baumannii (ATCC 19606) | 111 | 0.25 | 80.25 | 0.49 | 27.75 | 40.12 | 0.74 | Partial synergy |
Abbreviation: MIC, minimum inhibitory concentration.
a FICI was explained as fallows: ≤ 0.5 = synergistic; 0.5 - 0.75 = partial synergy; 0.76 - 1.0 = additive; 1.0 - 4.0 = indifferent; > 4.0 = antagonistic.
4.5.4. Time-Kill Assay
Figure 3 illustrates the time-kill assays on A. baumannii using Ib-AMP4 and Oncorhyncin II. Complete cell death was achieved within 11 hours for Ib-AMP4, 20 hours for Oncorhyncin II, and 5 hours for the combination of Ib-AMP4 and Oncorhyncin II. In contrast, Polymyxin E showed no effect on A. baumannii viability (Figure 3).
4.5.5. Growth Kinetic Assay
In the Growth Kinetic Assays, bacterial cultures were treated with twice the MIC concentrations of Ib-AMP4 and Oncorhyncin II. Throughout the experiment, the turbidity of the A. baumannii suspensions was routinely measured using a spectrophotometer. Notably, after 11 hours, both Ib-AMP4 and Oncorhyncin II independently achieved over a 95% reduction in bacterial culture turbidity. Remarkably, when Ib-AMP4 and Oncorhyncin II were used in combination, they demonstrated an even more rapid effect, reducing the turbidity of A. baumannii suspensions by more than 95% within just 7 hours. In contrast, Polymyxin E showed no significant impact on the turbidity of A. baumannii suspensions, as illustrated in Figure 4.
These findings highlight the potent antibacterial effects of Ib-AMP4 and Oncorhyncin II against A. baumannii. The observed synergistic effect of their combined application suggests a promising approach to more effectively inhibit bacterial growth. This study provides valuable insights for further research and the potential development of novel therapeutic strategies targeting A. baumannii infections.
5. Discussion
The rise of drug-resistant microorganisms has led to a significant increase in illness and mortality from bacterial infections, resulting in thousands of lives lost annually (16). This surge has heightened the urgency for alternative antibacterial agents. Among the most common nosocomial pathogens, multidrug-resistant (MDR) Acinetobacter baumannii has garnered considerable attention from researchers pursuing alternative treatments for infections commonly associated with Methicillin-resistant Staphylococcus aureus (MRSA) (17). This study focused on assessing the antibacterial effectiveness of two recombinant peptides, Ib-AMP4 and Oncorhyncin II, both individually and in combination (Ib-AMP4 + Oncorhyncin II), against A. baumannii infections.
Previous studies have demonstrated that the Ib-AMP4 and Oncorhyncin II peptides possess a broad spectrum of antibacterial properties, effectively inhibiting the growth of both gram-positive and gram-negative bacteria (18, 19). In the present research, these peptides were successfully expressed in E. coli and subsequently purified through nickel affinity chromatography, yielding highly potent forms that exhibited significant antimicrobial activity against A. baumannii.
Supporting prior findings, research has shown the antibacterial properties of these peptides. For instance, Fan X et al. demonstrated that Ib-AMP4 exerts its antibacterial effects by binding to bacterial cell membranes and disrupting membrane integrity, ultimately causing cell lysis (20). Similarly, Sadelaji et al. reported the rapid antibacterial potential of recombinant Ib-AMP4 in treating MRSA infections (21). Research by Jafari et al. confirmed Oncorhyncin II’s effectiveness in reducing MRSA cell growth and its positive effects in MRSA-infected mice treated with the peptide (7). Studies by Fernandes et al. have further illustrated that Oncorhyncin II, akin to other recombinant AMPs, exhibits remarkable antimicrobial efficacy against MRSA (22, 23).
In this study, MIC tests were conducted to evaluate the antimicrobial activity of the purified peptides against A. baumannii. The combination of Ib-AMP4 + Oncorhyncin II yielded the most effective inhibition results, demonstrating a higher level of bacterial killing power compared to the individual peptides. The time-kill results further supported the superior efficacy of the refolded recombinant Ib-AMP4 + Oncorhyncin II combination, which led to a rapid reduction in A. baumannii cell viability within the first 5 hours of exposure, outperforming the potency of each peptide individually. Additionally, the Growth Kinetic Assay indicated a notable decline in A. baumannii cell viability within the initial 7 hours of exposure to the combined peptides, highlighting the enhanced efficiency of the combination over individual peptide use.
Overall, all antimicrobial tests consistently demonstrated the significant antimicrobial activity of the recombinant Ib-AMP4 + Oncorhyncin II combination compared to the individual peptides. In summary, the synergistic action of Ib-AMP4 and Oncorhyncin II recombinant peptides showed a superior bacterial killing capacity in a shorter time compared to Polymyxin E, the control antibiotic. These results suggest that the combination of Ib-AMP4 and Oncorhyncin II peptides holds promise as an alternative therapeutic option for patients with A. baumannii infections. However, further clinical trials are needed to validate the efficacy of this treatment approach.
5.1. Conclusions
The primary objective of this investigation was to explore the combined effect of Ib-AMP4 and Oncorhyncin II antimicrobial peptides (AMPs) on A. baumannii (ATCC 19606), marking a pioneering effort in this area of research. Utilizing a variety of assays, including MIC, Checkerboard, Time-Kill, and Growth Kinetic tests, this study revealed that these peptides exhibit significant and rapid antibacterial activity against A. baumannii. These compelling findings highlight the potential of AMPs as novel antibiotics, either as standalone treatments or in combination with existing antibiotics, for combatting A. baumannii infections. To advance this research, the proteins studied—Ib-AMP4 and Oncorhyncin II—should be further evaluated in preclinical animal models. Moreover, additional clinical trials are essential to confirm their effectiveness in real-world settings.