1. Background
Malaria has a substantial global health impact, as this parasitic disease claims over 400 000 lives annually, primarily affecting children under the age of five. The World Health Organization's 2019 report indicated a slight rise in malaria cases, underscoring the ongoing challenge it poses to public health systems worldwide. The burden is especially severe in non-endemic countries undergoing ecological and climatic shifts, which intensify the spread of vector-borne diseases like malaria as a result of global warming. Regions such as Afghanistan, Somalia, Sudan, and Yemen are significantly impacted, collectively accounting for 95% of the global incidence (1-4). The World Health Organization (5) has established a gold standard for malaria surveillance, including precise case definitions, standardized data collection protocols, and systems for monitoring drug and vector resistance. These measures ensure a robust response to the evolving malaria threat (6).
However, the challenge is compounded by the vast diversity within Anopheles vector species and the influence of environmental factors on mosquito populations. This complexity is particularly evident in Iran, where Anopheles stephensi exhibits seasonal activity variations, complicating control efforts (4, 7, 8). The resurgence of malaria, driven by migration and climatic factors alongside concurrent health threats such as CRF35 AD, CCHF, and COVID-19, highlights the complex dynamics of disease control and underscores the role of imported cases in Iran’s epidemiological landscape from 2018 to 2023 (9-12). Further complicating control efforts in southeastern Iran are endemic challenges posed by Plasmodium vivax, exacerbated by a high prevalence of G6PD deficiency and thalassemia, which demand a nuanced approach due to their influence on disease resistance (13, 14).
Despite considerable progress, Iran continues to face persistent challenges in its pursuit of malaria elimination. These include societal obstacles in historically and newly affected areas (15), which require targeted control strategies and a thorough understanding of their unique characteristics and transmission dynamics. To effectively address this widespread health threat, active and attentive malaria monitoring through both passive and active surveillance methods remains essential.
2. Objectives
This research focuses on analyzing both urban and rural areas of Rask, monitoring the progression of malaria over five years from 2018 to 2023. The study aims to gain a comprehensive understanding of the impact of malaria in these regions.
3. Methods
3.1. Study Areas
Rask is located in the Sistan and Baluchistan province of southeast Iran. The city of Sarbaz is situated between 60°38' to 62°19' east longitude and 25°49' to 27°4' north latitude. As the center of Sarbaz, Rask lies at 61°24' east longitude and 26°14' north latitude, with an altitude of 410 meters above sea level. It borders Iranshahr and Meherstan to the north, Pakistan to the east, Chabahar to the south, and Nikshahar to the west (Figure 1) (16).
3.2. Data Collection
Data for this retrospective study was collected through the informatics system in Rask city and the Suburban Health Network. Malaria diagnoses were primarily established using rapid diagnostic tests (RDTs) (17), especially in areas with limited access to health centers. In more accessible regions, microscopic detection was employed, which involved examining blood smears as a diagnostic method. By utilizing both RDTs and microscopy, comprehensive coverage of malaria diagnoses across the study area was achieved. The informatics system gathered data and test results to compile an extensive dataset for the epidemiological analysis of malaria in Rask and its suburbs.
The complex interaction among different malaria foci—including newly active sites (previously unrecognized), transient sites, and established active sites—and their influence on disease prevalence requires a thorough understanding of malaria epidemiology. The uneven spatial distribution of malaria significantly impacts infection probabilities, thereby affecting disease transmission dynamics and population health outcomes. Furthermore, accurately determining reinfection rates in patients depends on distinguishing between relapses (re-emergence of dormant liver-stage parasites) and true reinfections by different strains of Plasmodium, which complicates malaria management and epidemiological analysis (18-20). Malaria cases were confirmed by microscopy or RDT kits (pLDH/HRP2). Comprehensive malaria diagnoses were conducted in multiple laboratories in Rask and the surrounding suburbs. Data were collected via a computerized system, and the analysis was based on patient characteristics and the specific Plasmodium species.
3.3. Statistical Analysis
To analyze associations between parasitic type and variables such as month, average temperature, and other factors, the study utilized the statistical package for the social sciences (SPSS), version 26.0. The analytical approach included analysis of variance (ANOVA) and the Tukey HSD post-hoc test. Results were reported as means ± standard deviations. Additionally, chi-square and Fisher's exact tests were applied to compare seroprevalence. Statistical significance was established at a P-value of less than 0.05.
4. Results
In 2023, a significant rise in infections was observed, with 1 669 cases reported, accounting for 51.1% of all cases. Table 1 provides a detailed summary of the findings. The predominant species identified during the study period was P. vivax, with a strong association (P < 0.001).
| Variables | 2020 | 2021 | 2022 | 2023 | P-Value |
|---|---|---|---|---|---|
| Number of malaria cases | 263 (83.7) | 237 (7.2) | 1100 (33.6) | 1669 (51.1) | - |
| Gender | 0.012 | ||||
| Male | 220 (83.7) | 197 (83.1) | 838 (76.2) | 1269 (50.3) | |
| Female | |||||
| Nonpregnant | 42 (16.0) | 40 (16.9) | 258 (23.5) | 386 (23.1) | |
| Pregnant | 1 (0.4) | 0 (0.0) | 4 (0.4) | 14 (0.8) | |
| Total population | 97884 | 100820 | 103844 | 106959 | |
| API (per 1000 population) b | 2.68 | 2.35 | 10.59 | 15.60 | |
| Age, y | 0.12 | ||||
| 0 - 4 | 8 (3.0) | 5 (2.1) | 34 (3.1) | 47 (2.8) | |
| 5 - 12 | 30 (11.4) | 31 (13.1) | 128 (11.6) | 200 (12.0) | |
| 13 - 18 | 25 (9.5) | 36 (1.1) | 167 (15.2) | 280 (16.8) | |
| 19 -30 | 102 (38.8) | 91 (38.4) | 380 (34.5) | 623 (37.3) | |
| 31 -50 | 80 (30.4) | 55 (23.2) | 279 (25.4) | 377 (22.6) | |
| > 51 | 18 (6.8) | 19 (8.0) | 112 (10.2) | 142 (8.5) | |
| Nationality | 0.006 | ||||
| Iranian | 232 (88.2) | 192 (81.0) | 897 (81.5) | 1321 (79.1) | |
| Pakistani | 30 (11.4) | 43 (18.1) | 185 (16.8) | 304 (18.2) | |
| Afghan | 1 (0.4) | 2 (0.8) | 18 (1.6) | 44 (2.6) |
Abbreviation: API, annual parasite incidence.
a Values are expressed as No. (%) unless otherwise indicated.
b API, a widely used indicator in malaria epidemiology that measures the number of new malaria cases per 1 000 people in a given year. API is calculated by dividing the total number of new malaria cases by the total population at risk, then multiplying by 1 000. This metric is critical in malaria surveillance and control efforts, as it helps assess transmission intensity and disease burden within specific areas. By tracking API, public health authorities can evaluate the effectiveness of malaria interventions and strategically allocate resources to high-risk populations and regions. High API values (typically above 10 - 30, depending on the area) may indicate regions with ongoing malaria transmission, where targeted control measures are necessary to reduce the disease burden.
As shown in Table 1, the distribution of malaria cases by gender was heavily skewed toward males (83.7% male cases vs. 16.0% female cases). The proportion of male cases was significantly higher than that of female cases during the first three years (P < 0.05), though no statistically significant difference was observed in the last two years. Among female cases, nonpregnant women consistently had a higher proportion of malaria cases compared to pregnant women throughout the study period. The proportion of pregnant women with malaria was low (less than 1% each year), and the differences across years were statistically insignificant (P > 0.05).
The distribution of malaria cases varied by age, with the highest proportion of cases observed in the 19 - 30 age group (Table 1). A statistically significant difference was noted in the distribution of malaria cases across years for the 0 - 4 age group (P = 0.12), with the proportion of cases in this group decreasing over time. Regarding nationality, Table 1 presents the distribution of malaria cases by nationality over the years. The majority of malaria cases were among Iranian nationals, with Pakistani nationals representing the second-highest proportion, followed by Afghan nationals with relatively low incidence. A statistically significant difference in the distribution of malaria cases by nationality was observed across the years (P = 0.006).
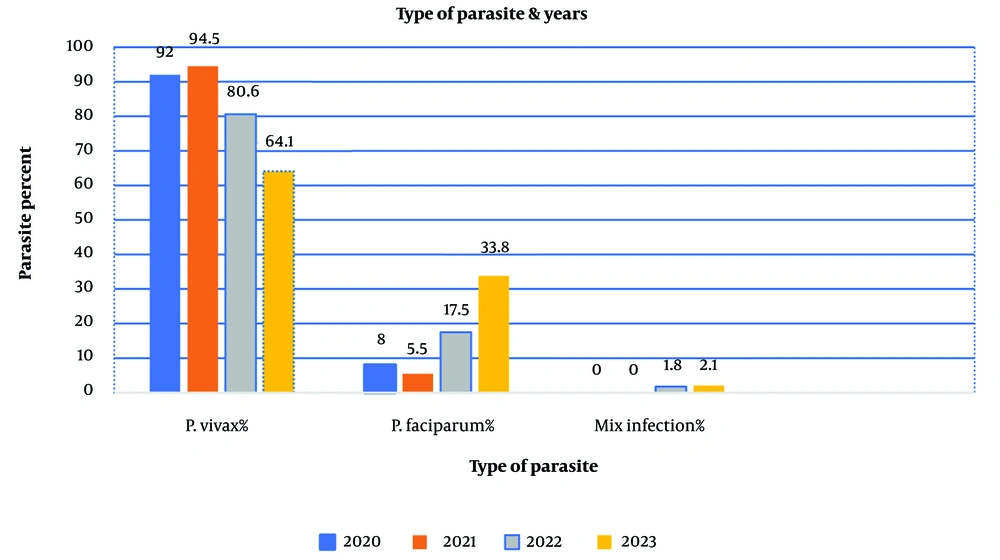
The P-value in this table is < 0.001, indicating a highly statistically significant difference in the distribution of malaria species over the years. Specifically, the proportion of P. vivax cases has declined over time, while P. falciparum cases have increased. Additionally, a small but consistent number of mixed infections were observed throughout the study. A P-value of < 0.001 suggests that these differences are highly unlikely to have occurred by chance, demonstrating statistical significance (Figure 2).
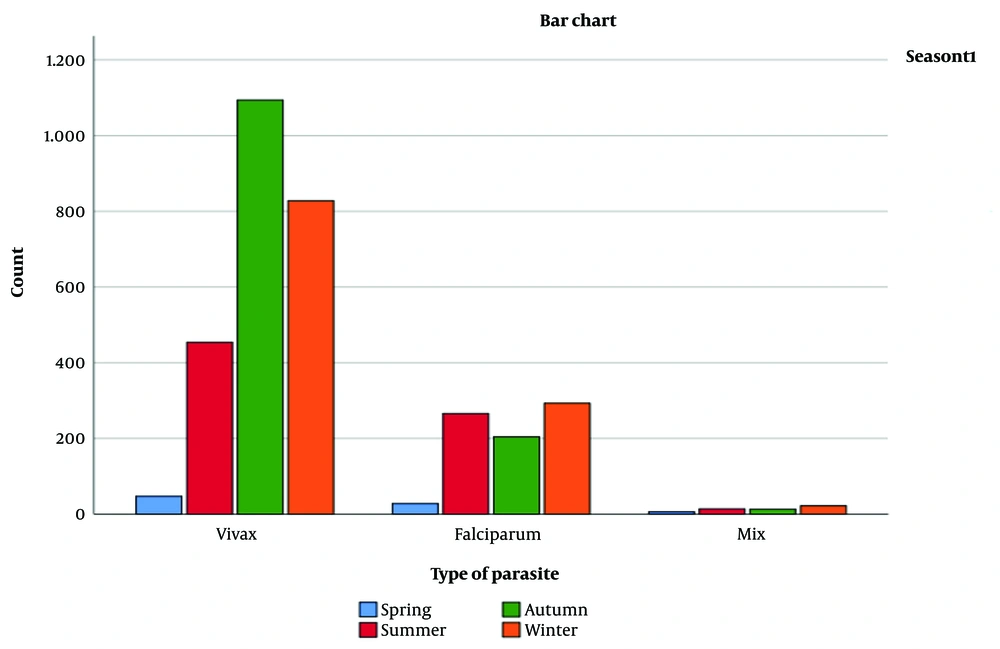
Table 2 presents the number of malaria cases by nationality and epidemiological transmission type (imported cases, cases transferred from imported cases, and indigenous cases). The correlation between nationality and transmission type was statistically significant (P-value < 0.001) based on Pearson's correlation coefficient. Additionally, the chi-square test confirmed a statistically significant association between nationality and epidemiological transmission (P-value < 0.001). Figure 3 displays the seasonal distribution of malaria cases by parasite type, showing the number of cases for each combination of season and parasite type (Table 3).
| Epidemiological Transmission | Nationality | Total | Correlation | P-Value | |||
|---|---|---|---|---|---|---|---|
| Iranian | Pakistani | Afghan | Pearson’s Correlation | P-Value | Chi2 | ||
| Import case | 1852 (75.4) | 540 (22.0) | 64 (2.6) | 2456 (100.0) | -0.222 | < 0.001 | < 0.001 |
| Transfer from import case | 733 (97.1) | 22 (2.9) | 0 (0.0) | 755 (100.0) | |||
| Indigenous | 57 (98.3) | 0 (0.0) | 1 (1.7) | 58 (100.0) | |||
a Values are expressed as No. (%) unless otherwise indicated.
| Month | Total Count | Vivax | Falciparum | Mix | P-Value |
|---|---|---|---|---|---|
| January | 41 | 23 (56.1) | 15 (36.6) | 3 (7.3) | < 0.001 |
| February | 16 | 13 (81.3) | 2 (12.5) | 1 (6.3) | 0.012 |
| March | 24 | 11 (45.8) | 11 (45.8) | 2 (8.3) | 0.545 |
| April | 273 | 145 (53.1) | 124 (45.4) | 4 (1.5) | < 0.001 |
| May | 309 | 202 (65.4) | 100 (32.4) | 7 (2.3) | < 0.001 |
| June | 152 | 107 (70.4) | 42 (27.6) | 3 (2.0) | < 0.001 |
| July | 152 | 132 (86.8) | 20 (13.2) | 0 (0.0) | < 0.001 |
| August | 439 | 363 (82.7) | 72 (16.4) | 4 (0.9) | < 0.001 |
| September | 720 | 599 (83.2) | 112 (15.6) | 9 (1.3) | < 0.001 |
| October | 478 | 377 (78.9) | 91 (19.0) | 10 (2.1) | < 0.001 |
| November | 520 | 308 (59.2) | 193 (37.1) | 19 (3.7) | < 0.001 |
| December | 145 | 95 (65.5) | 48 (33.1) | 2 (1.4) | < 0.001 |
| Total | 3269 | 2423 (74.1) | 791 (24.2) | 55 (1.7) | - |
a Values are expressed as No. (%) unless otherwise indicated.
Table 3 illustrates noticeable monthly trends in malaria type distribution, with significant variations. Specifically, P. vivax cases are more prevalent during the colder months (November to February), while P. falciparum cases increase during the warmer months (May to August). During the transition months (March, April, September, and October), there is a more balanced mix of malaria types.
| Condition of Disease Centre and Type of Parasite | Number of Cases |
|---|---|
| Before the occurrence of a positive case report | |
| Total | 3.269 |
| P. vivax | 2.423 |
| P. falciparum | 791 |
| Mix | 55 |
| Clean | |
| Total | 1.400 |
| P. vivax | 119 |
| P. falciparum | 20 |
| Mix | 1 |
| New active | |
| Total | 955 |
| P. vivax | 955 |
| P. falciparum | 363 |
| Mix | 31 |
| Old active | |
| Total | 853 |
| P. vivax | 853 |
| P. falciparum | 287 |
| Mix | 16 |
| New possibly transitory | |
| Total | 481 |
| P. vivax | 481 |
| P. falciparum | 119 |
| Mix | 7 |
This table presents the distribution of malaria cases based on two variables: Parasite type (P. vivax, P. falciparum, and mixed infections) and the condition of the disease center prior to a positive case report (categorized as "Clean," "New Active," "Old Active," and "New Possibly Transitory"). Cross-tabulating these variables reveals patterns in malaria distribution. For instance, the majority of P. vivax cases occurred in the "New Active" category (39.4%), while the majority of P. falciparum cases were found in the "Old Active" category (45.9%). This suggests potential differences in transmission dynamics, where P. vivax cases are more likely in newly active centers, and P. falciparum cases are more common in centers with a recent history of transmission.
The "Clean" category recorded the fewest cases across all parasite types, suggesting that maintaining a clean status at disease centers may be an effective strategy for reducing malaria transmission. Overall, this table offers insight into the distribution of malaria cases, highlighting patterns in the data that could inform targeted interventions.
Table 5 presents the distribution of malaria cases by parasite type—P. vivax, P. falciparum, and mixed infections—across different classifications of the disease center’s status after a positive report. The latest status is categorized into "New Active," "Old Active," and "New Possibly Transitory." Statistical analysis revealed a significant association between parasite type and disease center status (P-value = 0.002). The majority of P. vivax cases occurred when the disease center was "New Active" (43.3%) or "Old Active" (35.5%). For P. falciparum, cases were more evenly distributed across all categories, with the highest percentage occurring in the "New Active" status (48.9%). Notably, mixed infections were most frequently reported when the disease center was classified as "New Active" (58.2%). Table 5 provides the total case counts by parasite type and the center’s latest status. These findings underscore the importance of monitoring disease center status to understand malaria distribution and guide targeted intervention strategies.
| The Latest Status of the disease center after the positive report | Total | P. vivax | P. falciparum | Mix | P-Value |
|---|---|---|---|---|---|
| New active | 1.163 | 1.048 | 387 | 32 | 0.002 |
| Old active | 1.001 | 861 | 286 | 16 | |
| New possibly transitory | 575 | 499 | 116 | 7 | |
| Total | 2.740 | 2.408 | 791 | 55 |
5. Discussion
In Iran, malaria is stratified into four epidemiological regions, with the southern area being malaria-endemic (21). This study identified P. vivax as the predominant species, suggesting unique transmission dynamics and offering new research opportunities into its environmental interactions and health impacts. This finding is consistent with similar studies in Iran, such as one in Kermanshah, where P. vivax accounted for 98% of cases (22).
All areas were classified into four categories based on new autochthonous malaria cases per 1000 population: (1) there are four stages in malaria control efforts: (1) it is important to note that areas with an Annual Parasite Index/1000 more significant than five are designated as intensified control areas. This designation is substantial and requires attention to ensure the safety and well-being of individuals in those areas. By remaining informed and taking necessary precautions, we can work towards minimizing the impact of parasites and maintaining the health of our communities. (2) pre-elimination, where the API/1000 is between 5 and 1; (3) elimination, where the API/1000 is less than 1; and (4) prevention of reintroduction, for regions with no new autochthonous malaria cases in the past 36 months. Control measures, such as vector management and intervention strategies, are customized to each category. It's compelling to see how these targeted approaches enhance program effectiveness and implementation (4).
In our study, the API for malaria in the years 2021, 2022, and 2023 were 2.68, 2.35, 10.59, and 15.60, respectively, indicating an outbreak in this region of southeastern Iran. Several factors may contribute to the increase in API, particularly in southern or southeastern Iran: Climatic conditions: Climate change, including rising temperatures and shifts in rainfall patterns, can extend the mosquito breeding season and increase population density, leading to higher malaria transmission (23). The emergence of parasite resistance to antimalarial drugs, such as chloroquine, can cause treatment failures, thereby contributing to a rise in API (5). Mosquito resistance to insecticides can reduce the effectiveness of vector control measures, resulting in higher malaria transmission (24). Increased human movement across borders, particularly migration from malaria-endemic neighboring countries, can lead to the importation and spread of malaria (25). Limitations in healthcare resources can impede the efficient surveillance and treatment of malaria, resulting in elevated levels of the API (26). Poverty worsens the spread of malaria because of inadequate housing, lack of protective measures, and limited access to healthcare (27). Deforestation, irrigation, and changes in land use can create more suitable habitats for Anopheles mosquitoes, which carry malaria (28).
Soleimanifard et al. investigated 726 patients in Isfahan between 1859 and 2009. Among these, 679 patients (93.5%) were male, and 47 (6.5%) were female, with a mean age of 25.9 ± 9.9 years. Plasmodium vivax was identified as the most prevalent parasite, accounting for 94.6% of cases. The highest malaria prevalence was observed in 2005, with 243 patients (23.5%) during June (11.8%). Afghan immigrants made up 91% of the cases, while Iranian patients represented only 2.8%. Following treatment, 348 patients (47.9%) fully recovered, 39 patients (5.4%) remained under treatment, and there was no available information for 339 patients (46.7%) (29).
Recent studies indicate an increase in the Afghan refugee population in Iran. The United Nations High Commissioner for Refugees (UNHCR), a reliable source on refugee populations, reports that Iran hosts one of the world’s largest and most longstanding urban refugee populations, including Afghans who have fled conflict and economic instability in their home country. Recent events in Afghanistan have further intensified displacement towards neighboring countries, including Iran. A study by As highlights an outbreak in this southeastern region. Several factors may contribute to the increase in annual parasite incidence (API) for malaria, particularly in southern and southeastern Iran. Climatic conditions, such as rising temperatures and shifting rainfall patterns due to climate change, can extend mosquito breeding seasons and increase population density, potentially leading to higher malaria transmission rates (30).
In recent years, malaria incidence has increased in southeastern Iran, particularly in the provinces of Sistan and Baluchestan, Hormozgan, and Kerman. These trends are linked to changes in precipitation and humidity patterns in the region (31). A study conducted in Sistan and Baluchestan identified a noteworthy positive correlation between malaria incidence and average, minimum, and maximum monthly temperatures, along with a negative correlation with humidity (32). Similarly, research in Babol found the strongest positive correlation between malaria incidence and minimum air temperature (r = +0.991, P < 0.05) and the strongest negative correlation with relative humidity at noon (r = -0.863, P < 0.05) (33). Migration and weather conditions appear to be significant contributors to the recent rise in malaria cases in southeastern Iran.
The distribution of malaria cases also varies based on the type of parasite and the current status of the disease center, as shown in Table 5. Specifically, most P. vivax cases were reported when the disease center was classified as new active (43.3%) or old active (35.5%). In contrast, P. falciparum cases were more evenly distributed across all categories, with the highest percentage reported in newly active centers (48.9%). Most mixed infections were also reported in newly active centers (58.2%). This suggests that malaria type distribution may differ based on the disease center’s status, which has implications for targeted interventions. The association between parasite type and the latest status of the disease center was statistically significant (P = 0.002), indicating that the distribution of parasite types varies across the different categories of disease center status. This finding underscores the importance of monitoring the disease center’s status to better understand malaria distribution and inform targeted interventions.
The P-value indicates the probability that the observed differences in the data occurred by chance. In this case, a P-value of 0.002 suggests a very low probability (less than 0.2%) that the differences in malaria type distribution across the latest statuses of disease centers after a positive report occurred by chance. Thus, we can conclude there is a statistically significant difference in malaria type distribution across the latest statuses of the disease center following a positive report. The findings in Table 5 have important implications for malaria control and prevention. The variations in malaria type distribution based on the latest status of the disease center suggest that targeted interventions may be necessary to address the unique challenges of each parasite type. For instance, targeted indoor residual spraying or mass drug administration may be particularly effective in areas with higher P. vivax prevalence, while enhanced vector control measures may be more suitable for areas with high P. falciparum transmission. By monitoring the latest status of the disease center and adjusting interventions accordingly, malaria burden can potentially be reduced, leading to improved public health outcomes.
Figure 3 shows a higher incidence of malaria cases during the summer, consistent with previous studies reporting peak malaria transmission in warm and wet seasons. The proportion of P. vivax cases was highest in autumn, while P. falciparum cases peaked in summer. This aligns with previous research indicating that P. vivax is more prevalent in cooler, drier areas, whereas P.falciparum is more common in warmer, wetter regions. Figure 3 also illustrates an increase in mixed infections (P. vivax and P. falciparum) during the summer compared to other seasons. Mixed infections can be more complex to diagnose and treat, underscoring the importance of accurate diagnostics and appropriate treatment strategies.
The higher proportion of malaria cases in males compared to females, as shown in Table 1, aligns with previous studies in Iran and other malaria-endemic regions. This gender disparity may be due to varying exposure levels to malaria-carrying mosquitoes, with outdoor occupations that increase the likelihood of mosquito bites potentially contributing to higher exposure for males.
On the other hand, it is worth noting that the percentage of pregnant women affected by malaria is lower compared to that of nonpregnant women. This difference could be attributed to increased awareness and targeted prevention efforts specifically aimed at pregnant women. However, it is essential to recognize that even a relatively small number of malaria cases in pregnant women can have severe consequences for both the mother and the fetus. The lack of statistically significant differences in the proportion of pregnant women with malaria across different years may indicate that prevention efforts targeting pregnant women have been successful in maintaining low levels of malaria infection. Nonetheless, continuous monitoring and evaluation of these efforts are crucial to ensure long-term sustainability and effectiveness.
5.1. Conclusions
In conclusion, these findings highlight the ongoing need for surveillance and targeted interventions to mitigate the burden of malaria in the study area, particularly among high-risk populations such as males and nonpregnant women. The study had limitations related to data availability and potential sampling bias. Its location in Rask city, Iran, and reliance on specific diagnostic methods restricted the generalizability of the findings. Furthermore, external factors such as socioeconomic conditions, climate change, and vector control measures affecting malaria transmission were not explicitly taken into account in the study.