1. Background
Tooth decay is one of the most prevalent oral and dental disorders caused by bacterial excessive acid production; however, it also contributes to other conditions, such as cardiovascular disorders (CVDs) (1-3). Some of the most common decay-causing bacteria include Streptococcus mutans, S. mitis, S. sanguinis, S. salivarius, Actinomyces spp., Lactobacillus acidophilus, L. salivarius, L. casei, Peptostreptococcus spp., Staphylococcus spp., Eubacterium spp., Neisseria spp., and Micrococcus spp. Among these, the streptococci group is particularly common in the oral cavity (4). Of these, S. mutans is the most significant contributor to plaque formation and tooth decay. This Gram-positive bacterium promotes tooth caries by using the enzyme glucosyl transferase to convert sucrose into an extracellular polymer (4).
Streptococcus mutans colonizes tooth enamel by producing lipoteichoic acid. Bacterial attachment to the tooth surface and sugar breakdown lower the pH, leading to the deterioration of exterior tooth tissues, including enamel and dentine, which progresses into dental cavities (4, 5). In recent years, probiotics have gained popularity for their numerous health benefits for the gastrointestinal tract (GIT), urinary tract (UT), skin, and oral cavity. Numerous studies have assessed how oral probiotics affect conditions like tooth decay and oral infections (6-8). Probiotics boost the oral immune system, offering potential health benefits (9). The most advantageous oral probiotics for oral health include L. reuteri, L. salivarius, S. salivarius K12, S. salivarius M18, L. paracasei, and L. sakei (9-12).
Streptococcus salivarius K12 was the first commercially available probiotic. This strain produces bacteriocin-like inhibitory substances (BLIS) such as salivaricin A2 and salivaricin B, which have antibiotic properties. S. salivarius K12 reduces inflammation, blocks the NF-κB pathway, and prevents the generation and release of interleukin-8 (IL-8) (12). It inhibits several pathogens, including Actinomyces viscosus, A. naeslundii, S. agalactiae, S. pneumoniae, Enterococcus faecalis, Listeria monocytogenes, Haemophilus influenzae, Aspergillus coprophilus, and S. mutans. Another important probiotic strain, S. salivarius M18, also plays a significant role in promoting oral health (13). Prebiotics are short-chain carbohydrates that resist digestion by digestive enzymes and promote the growth and activity of probiotic species (14). Naturally, the antibacterial effects of probiotics are enhanced by prebiotics. Recent studies suggest that using probiotics can reduce or eliminate microbial resistance to antibiotics (14, 15). Bacteriocins, which are non-toxic, heat- and acid-resistant peptides, have been proposed as antibiotic substitutes. One notable example is nisin, a bacteriocin produced by L. lactis, which has demonstrated effectiveness against tooth decay (16).
Inulin, another prebiotic, is a soluble fiber that nourishes healthy bacteria but is not digested in the intestines. Inulin is found in numerous plants, such as chicory root, leek, onion, garlic, and asparagus. In addition to its benefits in oral health, inulin has been successfully shown to regulate gut microflora, supporting overall health (17, 18).
2. Objectives
The primary aim of the current study was to evaluate the invitro effects of the probiotics Streptococcus salivarius K12 and S. salivarius M18, as well as the prebiotics nisin and inulin, and their combination, on the growth of S. mutans.
3. Methods
3.1. Streptococcus mutans Bacterial Culture and Verification
In this study, each experiment was performed three times, and the results were calculated as the average of the three experiments. The standard strain of S. mutans ATCC 25175, provided by the Iranian Research Organization for Science and Technology (IROST), was cultured on Brucella agar base media supplemented with 5% defibrinated sheep blood, 5% horse serum, 5% vitamin K1, and 5% hemin. The culture was then transferred to brain-heart infusion (BHI) agar media. DNA extraction was followed by PCR amplification and sequencing methods to verify the bacterial strain using 16S rRNA-specific primers, as described previously by Shadkam et al. (19).
3.2. Prebiotics and Probiotics
The prebiotics nisin and inulin (Sigma-Aldrich, Germany) were prepared by serial dilution in physiological serum. Standard probiotic strains S. salivarius K12 ATCC BAA-1024 [DSM 13084] and S. salivarius M18 ATCC BAA-2593, also provided by the IROST, were obtained in lyophilized form and reactivated in the laboratory. These bacteria were repeatedly cultured on de Man, Rogosa, and Sharpe (MRS) agar media, and the final cultures were used for glycerol stock. Two 50 mL batches of MRS were inoculated separately with overnight cultures of S. salivarius K12 and S. salivarius M18. After incubation at 37°C until the optical density at 600 nm (OD600) reached 1, the cultures were centrifuged at 2000 g, and the supernatants containing bacteriocins were collected and stored at 4°C until use. In combination studies, the growth rate was calculated using the following formula:
This formula provided a normalized measure of cell proliferation, allowing for direct comparisons between the different treatment conditions.
3.3. Antibacterial Activity Assays
3.3.1. Microdilution Assay
In this study, the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays were performed. Briefly, S. mutans was prepared at a concentration of 100 CFU per well in 96-well microplates and incubated overnight at 37°C with the probiotics and prebiotics, both individually and in combination. The inhibition of bacterial growth was then assessed by measuring the absorbance at 600 nm using a microplate reader (Bio-Rad, USA).
3.3.2. Agar Overlay Interference Assay
In this assay, 10 µL of an active culture containing 1.5 × 10⁸ CFU/mL of each probiotic strain was spotted on MRS agar media and incubated at 37°C for 48 hours. Afterward, an active culture of S. mutans with a concentration of 1.5 × 10⁸ CFU/mL was inoculated into semi-solid BHI agar and poured onto the plates containing probiotics and prebiotics. The plates were incubated at 37°C for 24 hours, and the diameters of the clear zones around the spots were measured. The clear zones were categorized as weak (5 - 15 mm), moderate (15 - 25 mm), strong (25 - 35 mm), and very strong (> 35 mm) (20).
3.4. Agar Well Infiltration Assay
Initially, the indicator bacteria were cultivated for 24 hours in Mueller-Hinton broth (MHB) media. Then, 50 µL of their active cultures at half McFarland standard (1.5 × 10⁸ CFU/mL) were transferred onto the surface of Mueller-Hinton agar (MHA) plates. Wells with a diameter of 6 mm were created on the agar surface, and 100 µL of each probiotic and prebiotic solution was transferred into the wells. After being left to set at 4°C for 1 hour, the plates were incubated at 37°C for 24 hours. The clear zones surrounding the wells were measured in millimeters using a ruler (17). Positive results were defined as clear zones of 1 mm or larger.
3.5. Proteolytic Activities of the Probiotics
To verify the synthesis of bacteriocins by S. salivarius K12 and S. salivarius M18, an enzyme digestion assay was performed. Microbial suspensions were centrifuged at 2000 g for 15 minutes, and the pH of the media was adjusted to 7.1 using 5 M NaOH to eliminate any acidic inhibitory effects. The supernatants were then filtered using 0.22-µm filters. The microbial extracellular protease activity was assessed under three different pH conditions, as described by Thung (21). Briefly, 0.25 mL of cell-free supernatant (CFS) was mixed with 0.5 mL of buffer containing 0.5% (w/v) sulfanilamide azocasein (Sigma-Aldrich, USA) and incubated at 37°C for 30 minutes. The reaction was stopped by adding 0.75 mL of 10% (w/v) trichloroacetic acid (Merck, Germany) and incubating at room temperature (RT) for 30 minutes. After centrifugation at 16,000 g for 10 minutes, the supernatant was mixed with 0.6 mL of 1 M NaOH (Merck, Germany) and incubated for 15 minutes at room temperature before measuring the absorbance at 450 nm. An assay control was prepared by substituting the buffer for the CFS and substrate. The quantity of enzyme required to hydrolyze sulfanilamide-azocasein to produce a 0.001 change in absorbance per minute per milligram of protein is reported as one unit per milligram (U/mg) of specific protease activity.
3.6. Qualitative Proteolytic Activities of the Probiotics
The proteolytic activities of the probiotics were qualitatively identified using the skimmed milk hydrolysis method with minor modifications (21). A 10-hour logarithmic phase culture with a cell density of 10⁹ CFU/mL was used for the assessment. The culture was spotted onto agar media containing 2% (w/v) skim milk and incubated at 30°C for 48 hours. The values of bacterial colony diameters (X) and clear zone diameters (Y) were used to calculate the proteolytic index (Z) using the formula: Z = (Y - X)/X. Growth characteristics were assessed in liquid media containing 2% skimmed milk and 0% skimmed milk (control). Clear zones with diameters of 1 mm or larger were considered positive results. The proteolytic activity rates were recorded based on the diameters of the hydrolyzed clear zones as follows: - + for zones < 1 mm, - ++ for zones between 1 mm and 1.1 mm, - +++ for zones ≥ 1.1 mm.
3.7. Bile Salt and pH Tolerance Assessments of the Probiotics
To assess the resistance of the probiotics to bile salts (Oxgall) (Sigma-Aldrich, Germany) or biloxalate, two tubes were used—one containing MRS broth with 0.3% (w/v) bile salt, and the other containing 9 mL of MRS broth without bile salts (control). Briefly, 1% (90 µL) of fresh microbial culture in MRS broth was added to each tube and incubated at 37°C in anaerobic jars. Growth rates of the strains were assessed at 0 and 8 hours at 630 nm using a spectrophotometer. The resistance of the strains to bile salts was calculated using the inhibition coefficient (Cinh) formula. Strains with an inhibition coefficient of less than 0.4 were considered resistant to bile salts.
In this assay, bacterial suspensions were inoculated into MRS broth containing 0.3% biloxalate. Liquid culture media without biloxalate were used as a control. The optical density (OD) of the media was measured at 600 - 650 nm before incubation. After incubation at an appropriate temperature for 8 hours, the optical density was remeasured. Furthermore, the probiotic strains were assessed for acid tolerance. Microbial suspensions were inoculated into MRS media with pH values of 4, 5.2, and 7. After incubation at 37°C for 3 - 4 hours, dilutions of the inoculated media were prepared and recultured on agar media. The number of bacterial colonies was counted after incubation, ensuring that colony counts did not fall below 10⁶ CFU/mL (22). Strains that survived at pH 2 with a colony count not less than 10⁶ CFU/mL were reported as acid-resistant.
3.8. Statistical Analysis
Data were analyzed using SPSS version 18 (IBM, USA) and Excel 2016 (Microsoft, USA). Descriptive statistics were calculated for all variables, and data were expressed as mean ± standard deviation (SD). To assess the significance of differences between groups, either Student's t-test or ANOVA was used, depending on the data distribution and number of groups. For multiple comparisons, post-hoc tests, such as Tukey's HSD, were used to control for Type 1 errors. A P-value of < 0.05 was considered statistically significant.
4. Results
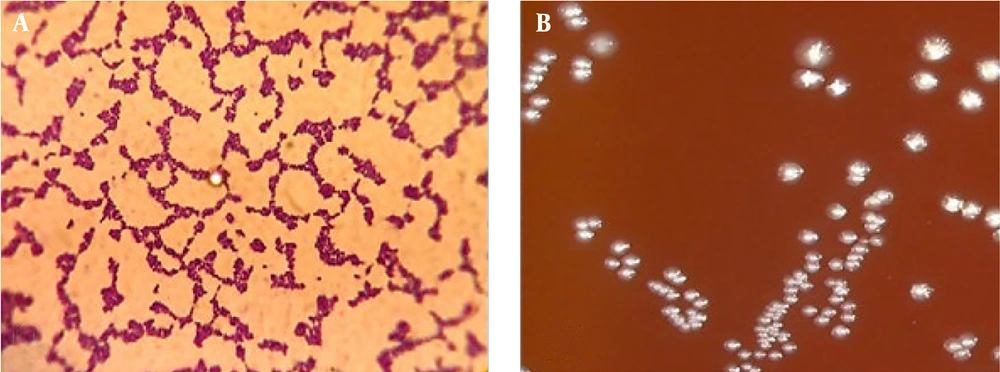
4.1. Macroscopic and Microscopic Characteristics of Streptococcus mutans
As a facultative anaerobic bacterium, S. mutans thrives in low oxygen environments. Its colonies on agar media were circular and white with distinct borders. The size of the colony varied significantly depending on the bacterial strain. Microscopically, S. mutans appeared elongated and stained gram-positive (Figure 1).
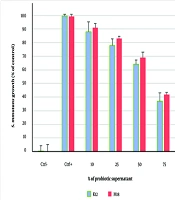
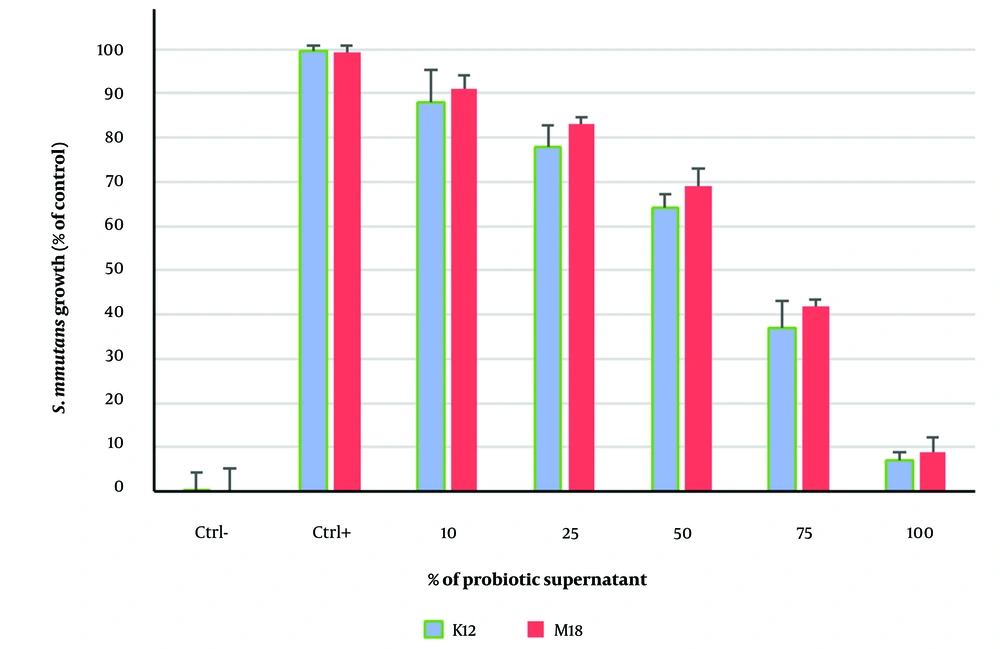
4.2. Minimum Inhibitory Concentration (MIC) of Streptococcus salivarius K12 and Streptococcus salivarius M18
In this assessment, S. mutans growth decreased by less than 50% at a concentration of 75%, making this concentration the MIC (Figure 2). The concentration of the probiotic supernatants that completely inhibited S. mutans growth was designated as the Minimum Bactericidal Concentration (MBC). Treatment with S. salivarius K12 significantly reduced growth compared to the control group (P < 0.05).
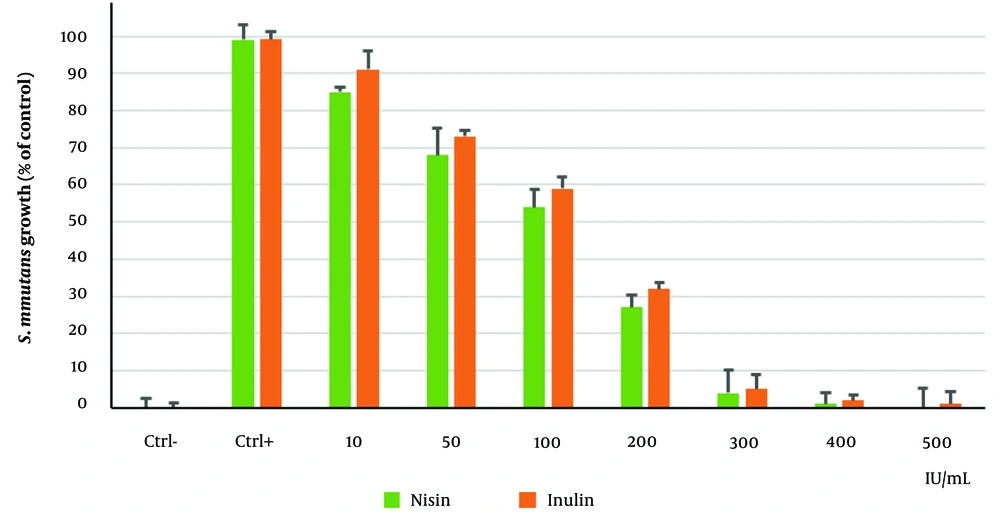
4.3. Minimum Inhibitory Concentration of Nisin and Inulin
In this experiment, S. mutans growth was inhibited by less than 50% at a concentration of 200 IU/ml, which was considered the MIC (Figure 3). At 300 IU/mL, nisin and inulin completely killed S. mutans, establishing this as the MBC. Treatment with nisin led to statistically significant reductions in bacterial growth compared to the control group (P < 0.05).
4.4. Agar Overlay Interference Assay
The results from this assay confirmed that S. salivarius K12, S. salivarius M18, nisin, and inulin exhibited antibacterial activity within 24 hours (Table 1). Treatments with S. salivarius K12 and nisin showed statistically significant reductions in bacterial growth rates compared to the other groups (P < 0.01).
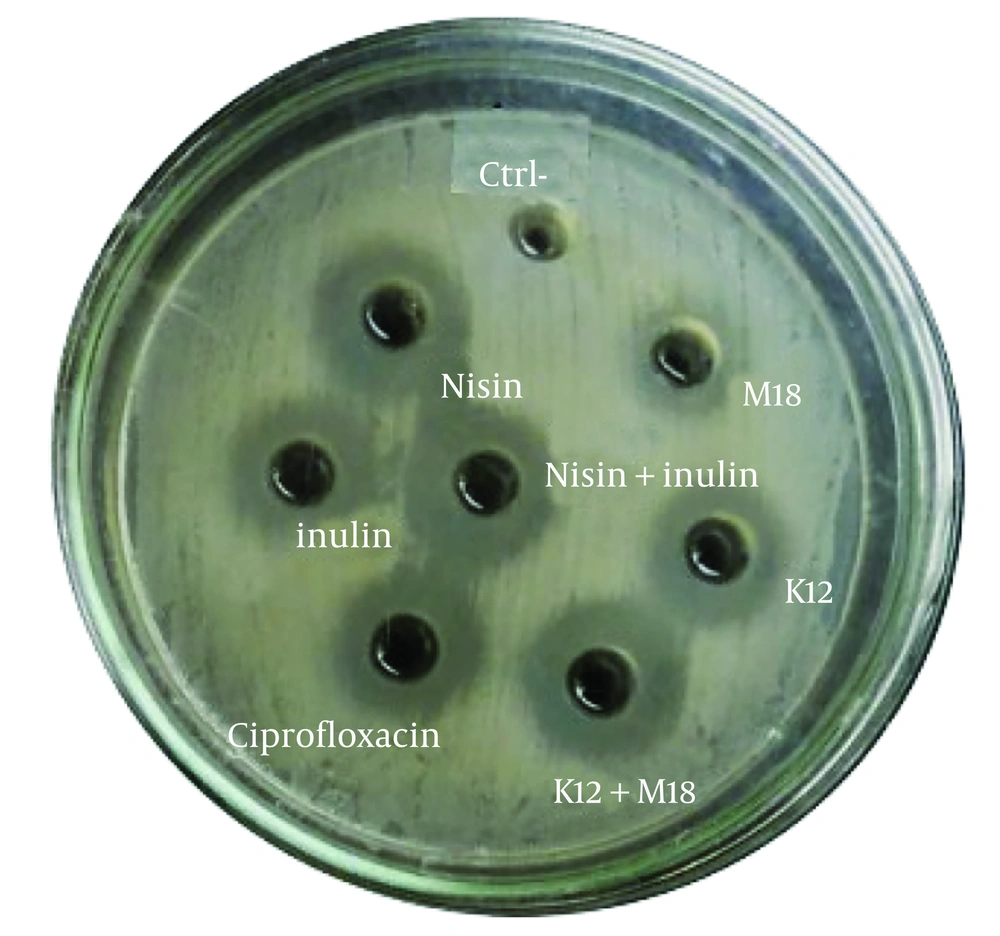
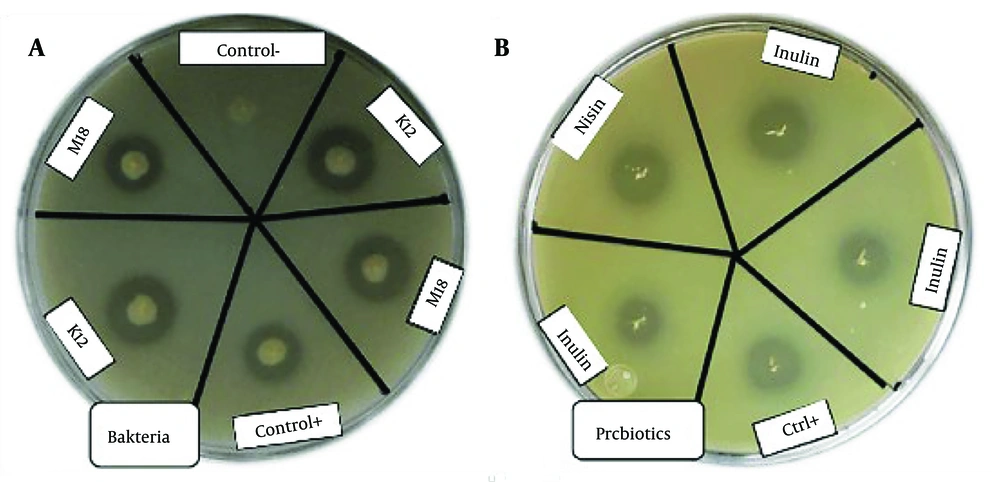
4.5. Antibacterial Activities of the Probiotics Using Agar Well Infiltration Method
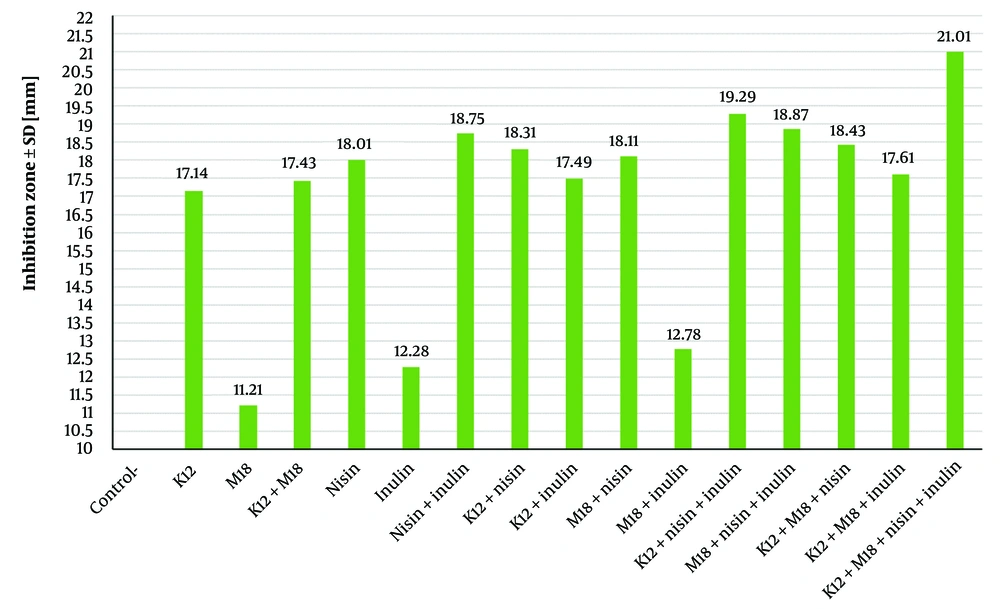
The antibacterial activity of the probiotics and prebiotics was assessed by measuring the clear zone diameters (CZD) of S. mutans colonies in the presence of these agents in BHI agar media (Figures 4 and 5). The clear zone diameters ranged from 11 to 21 mm, as shown in Table 2. The prebiotics generated larger clear zones around the S. mutans colonies compared to the probiotics. Streptococcus salivarius K12 showed significantly greater lethality against S. mutans compared to S. salivarius M18 (P = 0.0043), with a CZD of 17.14 mm ± 0.4 for K12 and 11.21 mm ± 0.3 for M18. Among the prebiotics, nisin exhibited a significantly larger inhibitory effect on S. mutans (CZD of 18 mm ± 0.01) compared to inulin (CZD of 12.28 mm ± 0.7) (P = 0.005).
| Test Group Agent | Diameter b |
|---|---|
| Negative control | 0.04 ± 0.05 |
| K12 | 17.14 ± 0.4 |
| M18 | 11.21± 0.3 |
| K12 and M18 | 17.43 ± 0.5 |
| Nisin | 18.01 ± 0.1 |
| Inulin | 12.28 ± 0.7 |
| Nisin and inulin | 18.75 ± 0.08 |
| K12 and nisin | 18.31 ± 0.4 |
| K12 and inulin | 17.49 ± 0.6 |
| M18 and nisin | 18.11 ± 0.5 |
| M18 and inulin | 12.78 ± 0.09 |
| K12, nisin and inulin | 19.29 ± 0.6 |
| M18, nisin and inulin | 18.87 ± 0.5 |
| K12, M18 and nisin | 18.43 ± 0.7 |
| K12, M18 and inulin | 17.61 ± 0.6 |
| K12, M18, nisin and inulin | 21.01 ± 0.03 |
a K12, Streptococcus salivarius K12; M18, Streptococcus salivarius M18.
b Diameter of transparent clear zone (mm).
The combination of the two prebiotics (CZD of 18.75 mm ± 0.08) displayed significantly greater inhibition of S. mutans compared to the combination of the two probiotics (CZD of 17.43 mm ± 0.5) (P = 0.031). The highest antibacterial activity was observed when all four agents (S. salivarius K12, S. salivarius M18, nisin, and inulin) were combined, producing a CZD of 21.01 mm ± 0.03, demonstrating their synergistic effects. These results aligned with those observed in the agar overlay interference assay, confirming the potent antibacterial activity of the combined probiotics and prebiotics against S. mutans.
4.6. Streptococcus salivarius Probiotic Potential
4.6.1. Tolerance to Bile Salts
The results demonstrated that both Streptococcus salivarius K12 and S. salivarius M18 exhibited tolerance to 0.3% bile salts. The growth halo diameters for K12 and M18 were 0.35 and 0.21, respectively, indicating that both strains were able to survive and grow in the presence of bile salts.
4.6.2. Tolerance to Acidic Conditions
Both S. salivarius K12 and S. salivarius M18 were able to survive under acidic conditions. Shortly after exposure to acidic environments, the growth halo diameters were 3.32 × 10⁹ for K12 and 1.07 × 10⁸ for M18. After 8 hours of acid exposure, a decrease in growth was observed, with the diameters decreasing to 1.04 × 10⁸ in K12 and 1.12 × 10⁷ in M18. Despite the reduction, both strains demonstrated considerable acid resistance.
4.7. Qualitative Evaluation of Proteolytic Activity
To validate the synthesis of bacteriocins by S. salivarius K12 and S. salivarius M18, the proteolytic activity of the probiotics and prebiotics was assessed in liquid culture media. Enzyme digestion was carried out using proteolytic enzymes, and bacterial suspensions were prepared for analysis. The pH of the bacteria-free supernatants was neutralized with NaOH to eliminate acidic inhibitory effects. However, it was found that the antibacterial activity was not due to bacteriocin production, as neutralized supernatants (pH 6.5) did not show inhibitory effects against S. mutans. Furthermore, the inhibitors produced by the probiotics were completely inactivated by the proteolytic enzyme trypsin, confirming their proteinaceous nature (Table 3). This finding indicates that the antibacterial effects observed in the study were not due to the bacteriocins produced by the probiotic strains, but rather due to other protein-based inhibitors.
| Probiotic strain | Supernatant (Control) | Neutralized Supernatant (pH 6.5) | Supernatant and Tripsin (1 mg/mL) |
|---|---|---|---|
| K12 | 0.88 ± 15 | - | 0.24 ± 13.4 |
| M18 | 0.29 ± 14 | - | 0.68 ± 12.9 |
a K12, Streptococcus salivarius K12; M18, Streptococcus salivarius M18
4.8. Quantitative Assessment of the Proteolytic Activity
The hydrolysis of skimmed milk was used to quantitatively assess the proteolytic activity of the probiotics. Both S. salivarius K12 and S. salivarius M18 demonstrated positive results, indicated by the presence of clear zones around the colonies (Figure 6). The radii of the clearing zones varied significantly, with S. salivarius M18 showing an average radius of 1.76 mm ± 0.08, while nisin exhibited the largest clearing zone with a radius of 4.45 mm ± 0.35 (Table 4). These results suggest that nisin had the highest proteolytic activity among the tested samples, followed by S. salivarius K12 and S. salivarius M18, indicating varying degrees of protein hydrolysis capabilities.
5. Discussion
The use of antimicrobial medications for treating tooth decay can lead to a range of issues, including allergic reactions, gastrointestinal side effects, and the development of bacterial resistance (23). With the growing public interest in alternative treatments for dental infections, there has been a corresponding increase in research on probiotics and prebiotics as potential solutions (1). The oral cavity is a highly complex ecosystem composed of numerous bacterial species, each playing distinct roles in oral health (24). Maintaining the balance of oral microflora can be achieved through probiotic microorganisms. In previous studies, dairy products have been used as carriers for probiotics, and their effects on reducing tooth decay and influencing the oral environment's pH have been explored (25). For instance, a 2011 study by Keller et al. showed that commercial probiotics reduced S. mutans populations in sucrose-containing laboratory samples (26). Probiotics prevent harmful bacterial colonization through several mechanisms, including competitive colonization, resource consumption, immune system modulation, and the production of toxins (27, 28).
Probiotics can produce antimicrobial compounds like bacteriocins, bacteriocin-like peptides, lactic acid (LA), and hydrogen peroxide (H₂O₂), which kill pathogens and positively influence host microbiota (29, 30). Various strains of lactic acid bacteria (LAB), including Streptococcus salivarius, produce bacteriocins that inhibit or kill pathogenic bacteria (31, 32). The present study demonstrated the antibacterial potential of probiotics, supporting findings from other research that indicates prebiotics such as nisin and inulin also possess antimicrobial properties (33-38). Specifically, the current study found that nisin and inulin inhibited S. mutans growth at a concentration of 200 IU/mL (MIC).
In a study by Akin et al. (2007), adding inulin to carrot juice significantly reduced pH and fungal growth in final products due to inulin's fermentation into short-chain fatty acids (SCFAs) (39). This reduction in pH contributed to inhibiting bacterial development. Furthermore, a study by Hagiwara et al. (2010) reported no adverse effects from feeding rats with nisin for 90 days, even at maximum doses of 225 mg/kg body weight per day. Importantly, nisin is the only prebiotic agent approved by the US Food and Drug Administration (US FDA) (38).
The current study reported clear inhibition zones ranging from 11 to 21 mm, with Streptococcus salivarius K12 demonstrating stronger inhibitory activity (17.14 mm) against S. mutans compared to S. salivarius M18 (11.21 mm) (P < 0.05). Nisin exhibited a greater inhibitory effect (18 mm) compared to inulin (12.28 mm) (P < 0.01). When combined, the prebiotics and probiotics showed enhanced effects, with the highest antibacterial activity recorded for a combination of all four agents (21.01 mm), indicating significant synergistic effects (P < 0.001). These results were consistent with those from the agar overlay interference assay.
Bile salt tolerance is generally considered essential for bacterial colonization and metabolic activity in the host’s small intestine (40). Hence, assessing the tolerance of probiotic strains to bile acids is a critical factor when evaluating their potential use. In this study, S. salivarius K12 and S. salivarius M18 demonstrated tolerance to bile concentrations of 0.3% and survival rates of 36% and 33%, respectively, which is consistent with findings from Boke et al. (41), who reported similar levels of bile salt tolerance in these strains.
5.1. Conclusions
In conclusion, the most critical outcome of this research was the discovery of synergistic effects between the prebiotics and probiotics, suggesting that their combination could be an innovative and effective approach for preventing tooth decay. These findings have practical implications for the development of new food supplements and anti-caries treatments, contributing to improved oral health strategies.