1. Background
The extensive impact of human papillomavirus (HPV) and associated diseases remains a significant public health concern (1, 2). According to the National Cancer Institute, HPV can lead to various cancers, including cervical, vaginal, vulvar, penile, anal, and oropharyngeal cancers (1). Additionally, HPV is linked to non-cancerous conditions such as anogenital warts and recurrent respiratory papillomatosis (3, 4). Over 200 HPV genotypes have been identified to date. Studies by the WHO and Colombian researchers have shown that 95% of individuals with cervical cancer tested positive for HPV DNA, with the 15 most common types—ranked by frequency—being HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73 (5, 6). Strong evidence links HPV to specific human cancers, including cervical cancer, head and neck tumors, penile carcinoma, oropharyngeal squamous cell carcinoma, and anal malignancies.
Globally, cervical cancer is the fourth most common cancer among women and is caused by HPV (7, 8). A recent meta-analysis examined HPV infection prevalence in healthy Iranian females and females with cervical cancer. The results showed HPV infection rates of 9.4% and 77.4% in the respective groups (7), with HPV-16 identified as the predominant genotype in both cases. In males, HPV-16 is also the primary genotype associated with penile cancer, while HPV-6 and HPV-11 are frequently observed in cases of genital warts (7, 9). Although limited research has focused on HPV genotype distribution among Iranian males, a recent study indicated an HPV prevalence rate of 55.7% in the sample population, with HPV-6 being the most commonly observed genotype (10).
According to the centers for disease control and prevention (CDC), approximately 1 in 9 men in the United States is thought to have an oral HPV infection. The prevalence is even higher—over 40%—among men who have sex with men. Human papillomavirus plays a pivotal role in sexually transmitted diseases (11). Conducting screenings and HPV genotyping in regions without HPV vaccination programs, especially in low- and middle-income countries, could substantially improve public health outcomes and help curb HPV transmission (12).
2. Objectives
This study aimed to determine the prevalence of HPV genotypes in both male and female populations in Southern Iran, providing a summary of the most common genotypes in the region. Among studies conducted across various provinces in Iran, this is, to the researchers' knowledge, the first survey in the country to utilize a four-sample approach—anal, saliva, tissue, and thin-prep (liquid-based cytology, used exclusively for female participants)—including both male and female subjects from both normal and external genital wart (eGW) populations. The findings from this study could be valuable for future research and public health initiatives, as well as for informing vaccine development and national vaccination strategies.
3. Methods
3.1. Study Population
This cross-sectional study included 82 females (71.9%) and 32 males (28.1%) from Southern Iran. The research focused on two groups: The first group consisted solely of women who visited Dena Hospital for routine annual gynecological check-ups, and the second group included both men and women who sought treatment for external genital warts at the Motahari Medical Center for HPV DNA testing and genotyping between April 2019 and March 2022. The subjects were predominantly sexually active, with ages ranging from 13 to 74 years.
Eligibility criteria required participants to be males and females over 14 years old who were sexually active and displayed clinical or subclinical signs of genital HPV. Exclusion criteria included individuals with immune system disorders such as HIV, those with weakened immune systems, and those taking immunosuppressive medications. Upon signing the informed consent form and completing the questionnaire, participants from both groups provided four types of samples: Urine, anal swab, biopsy of the wart or vaginal swab, and oral swab samples.
3.2. Exploring Samples
In this study, researchers aimed to examine the presence of HPV infection across various anatomical sites in both healthy women and those with eGW. Samples were meticulously collected by specialized clinicians to ensure accuracy. For both male and female participants, anal samples were obtained by an infectious disease specialist and a gynecologist. Participants were positioned on their left side, and clinicians retracted the buttocks to insert a moistened Dacron swab approximately 1.5 to 2 inches into the anus, targeting the junction of the anus and rectum where HPV-related lesions are commonly found (13). The clinician confirmed the swab’s positioning past the internal sphincter to ensure proper sample collection. The samples were then securely capped and transported to the virology laboratory for further analysis.
Female participants with genital warts, either clinically visible or subclinical, suggesting HPV infection were also included. These women underwent a gynecological examination, during which clinicians collected biopsy samples from the warts. During colposcopy, sterile brushes were used to scrape tissue samples from genital lesions located in the external genital area, vagina, and cervix, providing a thorough sampling from key areas of interest. Liquid-based cytology bottles were used to collect vaginal discharge samples from healthy women. These samples were transferred to a 1.5 mL tube, centrifuged at a specified speed and duration, after which the supernatant was discarded, and the concentrated pellet was resuspended in normal saline for HPV testing.
Urine samples were collected from all study participants. Each sample was washed with normal saline before centrifugation, and, following the removal of the supernatant, the pellet was resuspended and transferred to a 1.5 mL tube in normal saline. Additionally, scrapes and exfoliated cells from oral, anal, and vaginal samples were placed in separate vials containing a buffer solution consisting of tris-HCL 10mM (pH 8) and EDTA 1 mM. Each vial was labeled according to the anatomical site (i.e., genital, oral, or tissue). The vials were then sent to the Clinical Microbiology Research Center at Shiraz University of Medical Sciences in Iran and stored at -20°C until PCR testing.
Overall, this study employed rigorous methods and specialized techniques to ensure effective sampling and collection of biological materials from diverse anatomical sites for comprehensive analysis.
3.3. Human Papillomavirus DNA Detection
For DNA extraction from exfoliated cells in oral, anal, and vaginal samples, as well as urine, the RIBO-prep nucleic acid extraction kit (AmpliSens®, Moscow, K2-9-Et-100-CE, USA) was used. This involved centrifugation and sequential proteinase K digestion in accordance with the manufacturer’s instructions. To detect HPV DNA, two sets of standard primers, MY09/11 and GP5+/6+, were utilized in one-step real-time PCR assays, facilitating the identification of HPV infection.
The qPCRBIO SyGreen Mix Kit was used for the assay, with each reaction containing 5 µL of HPV DNA, 10 pmol of GP5+/6+ and MY11/MY09 primers (primer sequences provided in Table 1), and a total reaction volume of 20 µL. The PCR conditions included an initial heating at 94°C for 3 minutes, followed by 40 cycles at 95°C for 5 seconds, 55°C for 10 seconds, and 60°C for 30 seconds. After amplification, melt curve profiles were analyzed using the Applied Biosystems StepOnePlus™ RT-qPCR instrument (Thermo Fisher Scientific, Waltham, MA, USA).
3.4. Human Papillomavirus Genotyping
Human papillomavirus genotyping was performed on four swab samples using the Mehrviru HPV Genotyping Kit (01, 07005, 24E, 30 HPV Genotype, Iran). DNA extraction followed the manufacturer’s instructions. Briefly, 10 µL of extracted DNA was used in two separate 20 µL reactions with a specific set of primers. The assay utilized HPV-specific dual priming oligonucleotides for multiplex real-time polymerase chain reaction (PCR). This assay targeted 30 HPV types to simultaneously identify 12 high-risk (HR) HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), 7 probable/possible HR HPV types (HPV 26, 53, 66, 67, 68, 73, 82), and 11 low-risk (LR) HPV types (HPV 6, 11, 40, 42, 43, 44, 54, 61, 62, 89, 90).
3.5. Statistical Analysis
We estimated the prevalence of HPV infection and its genotypes using binomial distribution analysis, calculating the corresponding 95% confidence intervals (CIs). Statistical significance in comparing infection rates over time was set at a P-value < 0.05. All statistical analyses were conducted using SPSS software, version 17.0 (SPSS, IBM, USA).
4. Results
This study examined two distinct groups: The general population and a population with eGW. The mean age in the eGW group was 31.29 ± 9.1 years, slightly higher than the mean age of 29.67 ± 4.915 years in the general population. Among the participants, 61 (53.51%) disclosed their marital status, while 53 (46.49%) reported being single (Table 1). There was no significant difference in marital status disclosure between males and females. Approximately 42.98% of participants were within the 20 - 30 age range. Of the total sample, 79 individuals (69.3%) tested positive for HPV: 65 (81.3%) in the eGW group and 14 (41.2%) in the general population. A statistically significant difference was noted in the prevalence of HPV in oral samples between the two groups (P ≤ 0.1). In the eGW group, 61 participants had low-risk HPV (LR-HPV) genotypes, with 4 testing positive for HPV-18. In contrast, 14 individuals in the general population had LR-HPV genotypes, revealing significant differences in HPV positivity rates between the two groups.
| Characteristics | eGW (n = 80) | Normal (n = 34) | All (n = 114) |
|---|---|---|---|
| Age (y) | |||
| ≤ 20 | 8 (10) | 1 (2.94) | 9 (7.89) |
| 20 - 30 | 34 (42.5) | 15 (44.11) | 49 (42.98) |
| 30 - 40 | 23 (28.75) | 14 (41.17) | 37 (32.45) |
| ≥ 40 | 15 (18.75) | 0 (0) | 15 (13.15) |
| Gender | |||
| Male | 32 (40) | 0 | 32 (28.07) |
| Female | 48 (60) | 34 (100) | 82 (71.92) |
| Habitation | |||
| City | 63 (78.8) | 34 (100) | 97 (85.1) |
| Village | 17 (21.3) | 0 | 17 (14.9) |
| Marital status | |||
| Single | 53 (66.25) | 8 (23.53) | 61 (53.51) |
| Married | 27 (33.75) | 26 (76.47) | 53 (46.49) |
| Education | |||
| Middle School | 6 (7.8) | 0 | 6 (5.4) |
| Diploma | 33 (42.9) | 10 (29.4) | 43 (38.7) |
| College | 38 (19.4) | 24 (70.6) | 62 (54.4) |
| Job | |||
| Housewife | 12 (15) | 17 (50) | 29 (25.4) |
| Employed or Freelance Job | 58 (72.5) | 17 (50) | 75 (65.8) |
| Addiction | |||
| Cigarettes | 10 (12.5) | 0 | 10 (8.77) |
| Alcohol | 6 (7.5) | 4 (11.76) | 10 (8.77) |
| Other | 28 (35) | 9 (26.47) | 37 (32.45) |
| Number sexual partners | |||
| 1 | 23 (28.75) | 34 (100) | 57 (50) |
| 2 - 4 | 35 (43.75) | 0 | 35 (30.7) |
| 5 < | 20 (25) | 0 | 20 (17.5) |
| Using condom | |||
| Yes | 25 (31.25) | 25 (73.5) | 50 (43.9) |
| No | 55 (68.75) | 9 (26.5) | 64 (56.1) |
| sexual relations | |||
| Vaginal | 16 (20) | 27 (79.4) | 43 (37.7) |
| Other | 62 (77.5) | 5 (14.7) | 67 (58.8) |
| High risk history | |||
| Positive | 38 (47.5) | 0 | 38 (33.3) |
| Negative | 42 (52.5) | 34 (100) | 76 (66.7) |
| Sexual partners | |||
| Opposite gender | 76 (97.4) | 34 (100) | 110 (98.2) |
| Both gender | 2 (2.6) | 0 | 2 (1.8) |
| Age first sexual | |||
| < 15 | 5 (6.3) | 0 | 5 (4.4) |
| 15 - 20 | 26 (32.9) | 9 (26.5) | 35 (31) |
| 20 - 25 | 32 (40.5) | 14 (41.2) | 46 (40.7) |
| 25 - 30 | 16 (20.3) | 11 (32.4) | 27 (23.9) |
| Child birth | |||
| Natural | 10 (12.5) | 3 (8.8) | 13 (11.4) |
| Cesarean | 5 (6.25) | 9 (26.5) | 14 (12.3) |
| Contraception | |||
| Natural | 14 (17.5) | 7 (50.6) | 21 (18.4) |
| Condom | 12 (15) | 15 (44.1) | 27 (23.7) |
| Vaginal HPV PCR | |||
| Positive | 0 | 5 (14.7) | 5 (4.4) |
| Negative | 0 | 29 (85.3) | 29 (25.4) |
| Tissue HPV PCR | |||
| Positive | 64 (80) | 0 | 64 (56.1) |
| Negative | 10 (12.5) | 0 | 10 (8.8) |
| Oral HPV PCR | |||
| Positive | 18 (22.5) | 2 (5.9) | 20 (17.5) |
| Negative | 54 (67.5) | 32 (94.1) | 86 (75.4) |
| Anal HPV PCR | |||
| Positive | 19 (23.75) | 8 (23.5) | 27 (23.7) |
| Negative | 55 (68.75) | 26 (76.5) | 81 (71.1) |
| Urine HPV PCR | |||
| Positive | 11 (13.75) | 6 (17.64) | 17 (14.9) |
| Negative | 47 (58.75) | 28 (82.36) | 75 (65.8) |
| HPV PCR | |||
| Positive | 65 (81.25) | 14 (41.2) | 79 (69.3) |
| Negative | 15 (18.75) | 20 (58.8) | 35 (30.7) |
| HPV genotype | |||
| None High Risk | 61 (76.25) | 14 (41.2) | 75 (65.8) |
| Genotype 18 | 4 (5) | 0 | 4 (35) |
Characteristics of Participants Related to Sociodemographics, Lifestyle, and Sexual Behavior a
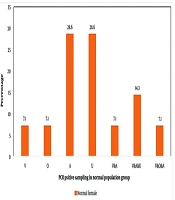
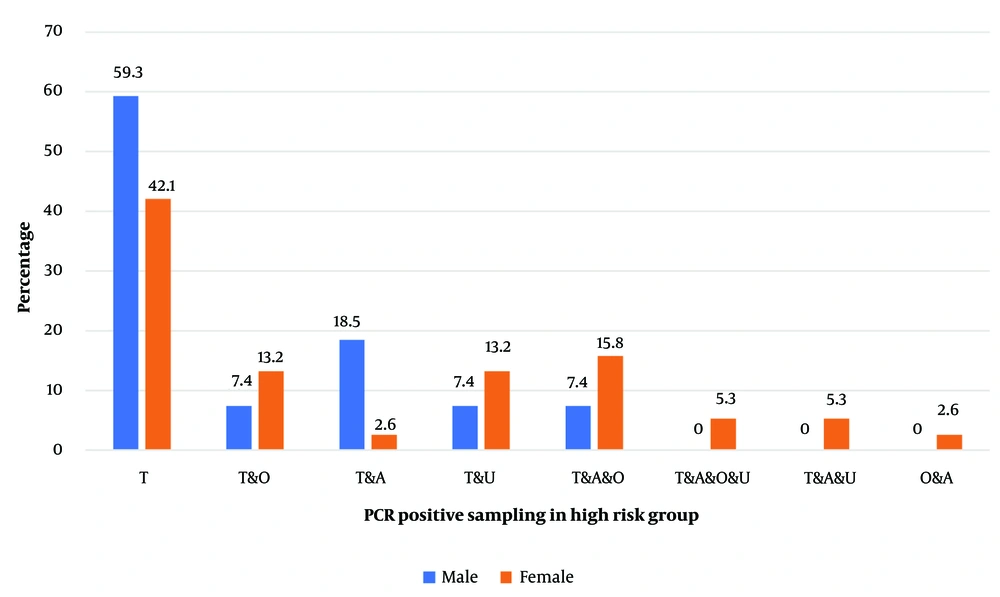
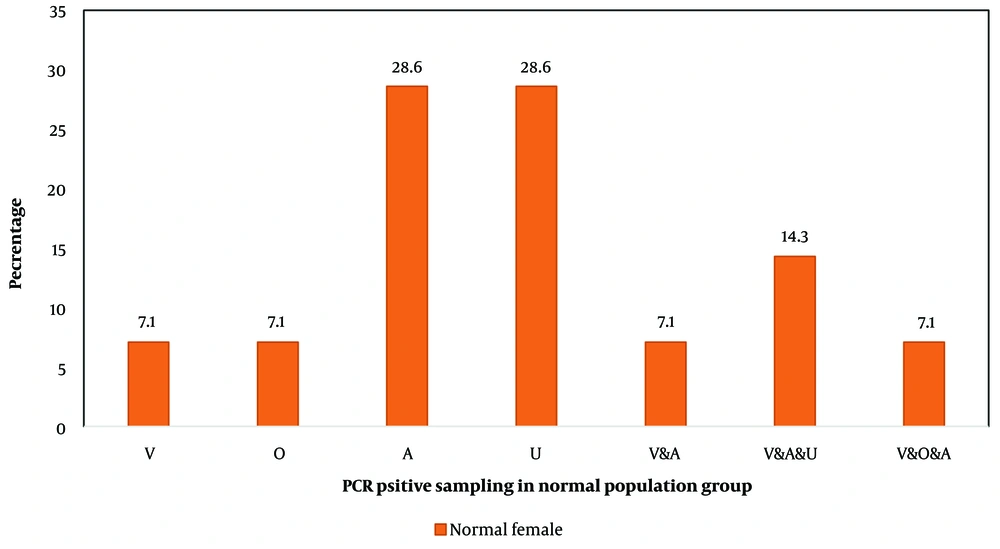
The male participants in the eGW group exhibited the highest rates of HPV positivity in both anal and tissue samples: 18.5% tested positive in the anal samples, while a much higher 59.3% tested positive in tissue samples. Among female participants in the eGW group, the highest positivity rates were observed in anal, oral, and urine samples, with positivity ranging approximately from 15% to 2% (Figure 1). Notably, among females in the general population, an unusually high proportion of HPV-positive results (around 29%) was seen in anal and urine samples compared to vaginal samples (Figure 2).
The percentages represent the proportion of individuals in the eGW group who tested positive for HPV in each sample type. Anal (A): Human papillomavirus (HPV) detected in anal swabs; urine (U): HPV detected in urine samples; tissue (T): HPV detected in Wart tissue; oral (O): HPV detected in oral swabs. The frequency of the number of samples in one person is expressed as; V & A: Vaginal and anal samples; V & A & U: Vaginal, anal, and urine samples; V & O & A: Vaginal, oral, and anal samples
The percentages represent the proportion of individuals in the normal population group who tested positive for human papillomavirus (HPV) in each sample type. Anal (A): HPV detected in anal swabs; urine (U): HPV detected in urine samples; tissue (T): HPV detected in Wart tissue; oral (O): HPV detected in oral swabs. The frequency of the number of samples in one person is expressed as; V & A: Vaginal and Anal samples; V & A & U: Vaginal, anal, and urine samples; V & O & A: Vaginal, Oral, and Anal samples.
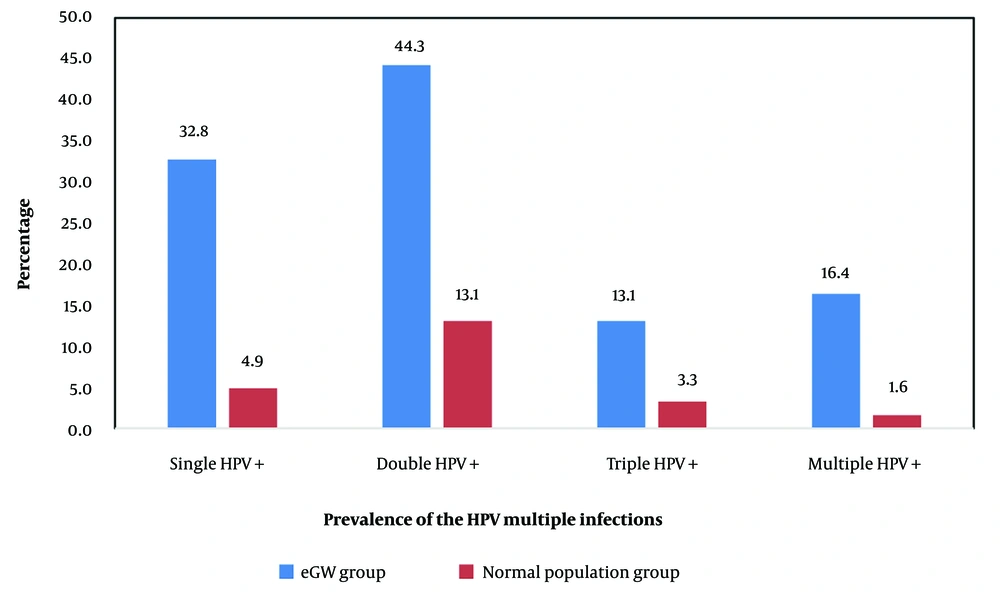
Among those who tested positive in anal samples, 27 individuals had a positive result, with 14 (51.85%) reporting a history of anal intercourse; 13 of these cases were in the eGW group. Of the 20 positive oral sample cases, 70% reported a history of oral sex. The study also analyzed various HPV genotypes: Single-type infections (20 cases with types 18, 6, 40, 90, and 35), double-type infections (27 cases with types 6/40, 6/59, 6/26, and 6/68), triple-type infections (8 cases with types 6/40/59, 6/40/53, and 6/40/18), and multiple-type infections (10 cases with types 6/40/59/67 and 68/26/53/6/44). As shown in Figure 3, co-infection with two HPV types was more common or detected more frequently than single or multiple-type infections.
5. Discussion
There is limited reliable data on the prevalence of HPV among outpatients in Iran. This investigation primarily focused on recently collected samples referred to reputable laboratories in Shiraz. The observed increase in HPV cases has occurred regardless of age, marital status, or economic background. This notable rise in cases can be attributed to two main factors: (1) Limited sexual education among the younger population, and (2) restricted access to HPV vaccines within the country. In various nations, the rise in HPV infections has been linked to earlier onset of sexual activity and changes in sexual behavior, such as multiple sexual partnerships and insufficient preventive measures (14). Undoubtedly, these factors may also contribute to the trends observed within the Iranian population.
Recently, there has been growing interest in HPV infections beyond the cervix. Human papillomavirus is suggested to play a carcinogenic role not only in cervical cancer, where it is a key factor, but also in anal and oropharyngeal cancers. Nearly all cervical cancer cases globally are associated with high-risk HPV (15, 16). Therefore, identifying HPV infections and genotyping them in clinical cases is essential. In this study, conducted at two prominent academic health centers in Shiraz, Iran, we found significant differences in HPV prevalence between individuals with eGW and the normal population. Overall, the prevalence of HPV among four types of samples from eGW individuals was approximately twice that of the normal population.
One study found a relatively high prevalence of HPV among both males and females, with rates of 55.7% for men and 52% for women. Previous studies in various countries have reported HPV prevalence among females ranging from 5.5% to 57.4%, with variations based on study populations and methods. Among Iranian males, four studies reported HPV prevalence rates between 9.5% and 54% (10). In a 2017 study conducted in Kermanshah, Iran, among 87 samples examined, 62.1% showed signs of HPV (17). Human papillomavirus infection was identified in 52% of inflammatory cases, 37% of genital wart cases, 9.3% of cervical cancer cases, and 1.8% of normal cases out of a total of 54 samples (7). In a 2019 study conducted in Tehran, which included 10,266 individuals from both genders across all 31 provinces in Iran, the findings revealed a high HPV prevalence rate of 49.5% (7).
Our study revealed a notably higher HPV positivity rate among both the normal population and eGW groups compared to previous studies. Globally, the highest burden of HPV infection is estimated in African and Latin American countries, while southern Europe and Southeast Asia report the lowest prevalence (18). The results indicated that 67.1% of patients tested positive for high-risk HPV (HR-HPV) genotypes, while 32.9% had low-risk HPV (LR-HPV) genotypes. In a study by Chalabiani et al., the rate of HR-HPV was also reported to be 67.2% across various regions of Iran. In our study, the age group with the highest incidence of HPV infection among women was those under 30 years old, while most women with both low-risk (LR) and high-risk (HR) genotypes were over 30 years old (19). Additionally, our findings revealed that the proportion of HPV positivity in tissue samples was significantly higher among men compared to women.
In another study examining positivity rates in various sample types among women with genital warts, anal, urine, and oral samples showed higher positivity rates compared to other sample types. Similarly, among normal women, LR-HPV infection rates in anal and urine samples were higher than in other samples. Anal cancer is the second most common HPV-related cancer after cervical cancer, with approximately 88% of anal cancers attributed to HPV, particularly HPV16 (20, 21). The incidence of anal cancer has been increasing over recent years, especially among high-risk populations of both men and women (22). Although HPV can be transmitted from men to women during anal sex (22, 23), a statistically significant association between anal sex and anal HPV infection prevalence is often undetected. In our study, none of the women in the normal group reported engaging in anal sex, which may be due to cultural taboos surrounding this practice in our country (24).
Few studies have examined the prevalence of both cervical and anal HPV infections among women, with some finding that anal HPV infection is as common, if not more common, than cervical HPV. Prior research has indicated that women in the general population have higher prevalence rates of anal HPV infection for any HPV type. Additionally, anal cancer appears to disproportionately impact women, with a 50% higher incidence rate in females than in males (24).
This study provides valuable insights into the demographics and HPV prevalence among both the general population and a group affected by genital warts. The findings reveal higher rates of HPV positivity, particularly in the genital wart group, suggesting a potential link between genital warts and HPV infection. The observed disparity in HPV presence in oral samples underscores the importance of exploring HPV transmission through various routes. Research on HPV genotyping in cervical cancer suggests that the distribution of HPV genotypes varies by race and geographic location (25). Therefore, country-specific epidemiological data on HPV genotypes can be instrumental in developing effective strategies to prevent cervical cancer. According to our findings, 35% of patients had high-risk HPV (HR-HPV) genotypes, while 65.8% had low-risk HPV (LR-HPV) genotypes. In the normal population, all detected genotypes were LR-HPV. However, in the eGW group, four individuals were found to have HR-HPV type 18.
A study by Chalabiani et al. reported the HR-HPV prevalence across different regions of Iran at 67.2%. In this study, the age group with the highest prevalence of women having both low-risk (LR) and high-risk (HR) HPV genotypes simultaneously was under 30 years old, with HPV infection also being most common in those under 30 (19). Similarly, findings from Shalchimanesh et al.'s study indicated a higher prevalence of HR-HPV than LR-HPV in the investigated population. Among 219 participants, 147 (67.1%) were found to have high-risk genotypes, while 72 (32.9%) had low-risk genotypes. The differences in high-risk genotype percentages between our study and other studies in Iran may be due to variations in the genotypes investigated (26). Our research focused on HR-HPV genotypes 16, 18, 31, and 33, while other studies included additional HR-HPV genotypes such as 53, 39, and 68, which could explain some of the observed variation.
Further research is needed to deepen our understanding of the association between HPV infection, genital warts, and sociodemographic factors. Additionally, long-term follow-up studies may offer insights into the potential progression of HPV infection in individuals with genital warts and its impact on overall health outcomes.
5.1. Conclusions
Significant differences in HPV prevalence were observed between individuals with eGW and those in the normal population. Overall, the prevalence of HPV across four sample types from eGW individuals was approximately double that observed in the normal population. However, further research is needed to clarify the relationship between HPV infection, eGW, and various sociodemographic factors. Additionally, long-term follow-up studies could provide valuable insights into the potential progression of HPV infection in individuals with genital warts and its broader impact on health outcomes.