1. Introduction
Chlamydia psittaci is an obligate intracellular Gram-negative pathogen that typically infects birds and can also infect humans, causing psittacosis (1). Chlamydia psittaci exists in two states: Elementary bodies (EBs) and reticulate bodies (RBs). Elementary bodies are smaller in size (0.2 - 0.4 μm), biologically inactive, extracellular, non-replicative, and infectious. Reticulate bodies are larger (0.7 - 1.5 μm), metabolically active, replicative, but noninfectious. During the transformation process between these states, inclusion bodies are formed, which change color from red to purple after Giemsa staining (2). A history of contact with poultry is considered a major risk factor for infection; however, there are increasing reports that C. psittaci infection is possible even without such a history. It can infect humans through the respiratory tract in the form of air or aerosols, or through human skin, mucous membranes, and the digestive tract via excrement carrying pathogens (3).
Psittacosis is not a common disease, but its distribution is widespread, with reports in the United States, Europe, Australia, Japan, and China (4). The clinical manifestations of patients with C. psittaci infection are atypical, and depending on the patient's immune function, they can range from asymptomatic to fatal systemic manifestations (5). Additionally, community-acquired pneumonia caused by C. psittaci infection accounts for approximately 1 - 2% annually (6). Clinical symptoms of C. psittaci pneumonia usually include fever, dry cough, headache, fatigue, myalgia, and chest tightness, with some cases presenting with rapidly progressing severe pneumonia, acute respiratory distress syndrome, and multi-organ failure (6). Due to the presence of asymptomatic patients and the lack of specific manifestations, the diagnosis of C. psittaci infection is often delayed (7). If left untreated, it may lead to more serious infections, including sepsis and meningitis, which can be fatal (8-10). Therefore, quickly and accurately diagnosing C. psittaci is a major challenge in clinical practice. We report a case of severe pneumonia caused by C. psittaci, aiming to provide a reference for the diagnosis and treatment of C. psittaci infection.
2. Case Presentation
2.1. Clinical Features
A female patient in her 60s presented with fever, accompanied by cough and sputum, five days prior to seeking medical attention at a local hospital. Examination revealed an increased white blood cell count and elevated high-sensitivity C-reactive protein levels. Chest CT showed inflammatory changes in the lower lobe of the right lung. After two days of anti-infective treatment with tazobactam sodium combined with levofloxacin, there was no significant improvement, and the patient's cough and sputum worsened, accompanied by symptoms of chest tightness and shortness of breath. Consequently, the patient sought further treatment at our hospital.
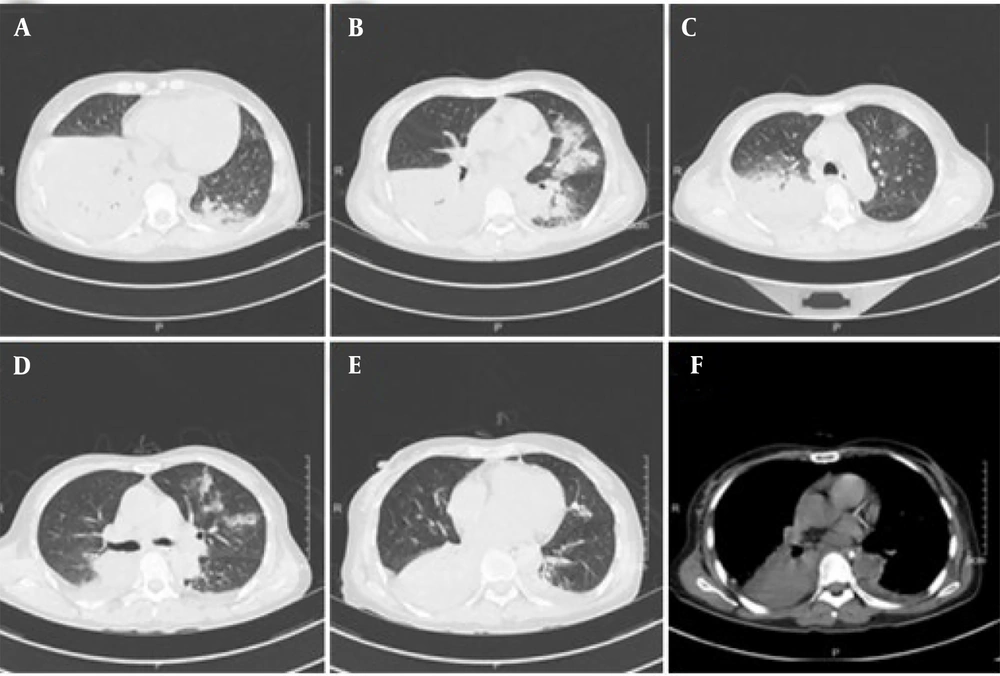
The patient had a previously healthy constitution, with no underlying diseases or long-term medication history. Upon admission, relevant examinations were conducted. The patient's temperature was 38°C, pulse rate was 115 beats per minute, respiration rate was 20 breaths per minute, blood pressure was 158/90 mmHg, and blood oxygen saturation was 92%. Auscultation revealed thick breathing sounds and moist rales in both lungs. Laboratory tests indicated elevated levels of infectious markers and inflammatory cytokines, as well as abnormal liver function (Table 1). A chest CT scan showed patchy and flocculent shadows in both lungs, with blurred boundaries and consolidation in the lower lobe of the right lung, suggesting inflammation (Figure 1).
| Laboratory Indicators | Result | Reference Range |
|---|---|---|
| Blood pH | 7.475 | 7.350 - 7.450 |
| Partial pressure of oxygen, mmHg | 62 | 80 - 100 |
| Partial pressure of carbon dioxide, mmHg | 25.8 | 35.0 - 45.0 |
| White blood cell count, × 109/L | 13.30 | 3.50 - 9.50 |
| Neutrophil count, × 109/L | 12.82 | 1.80 - 6.30 |
| C-reactive protein, mg/L | 258.3 | ≤ 10.0 |
| Serum total protein, g/L | 50.5 | 65.0 - 85.0 |
| Serum albumin, g/L | 22.2 | 40.0 - 55.0 |
| Glutamic-pyruvic transaminase, U/L | 79 | 7 - 40 |
| Glutamic oxaloacetic transaminase, U/L | 126 | 13 - 35 |
| Procalcitonin, ng/mL | 4.20 | ≤ 0.50 |
| Interleukin-6, pg/mL | 6.97 | ≤ 5.00 |
| Interferon-γ, pg/mL | 26.25 | ≤ 6.00 |
2.2. Etiological Examination
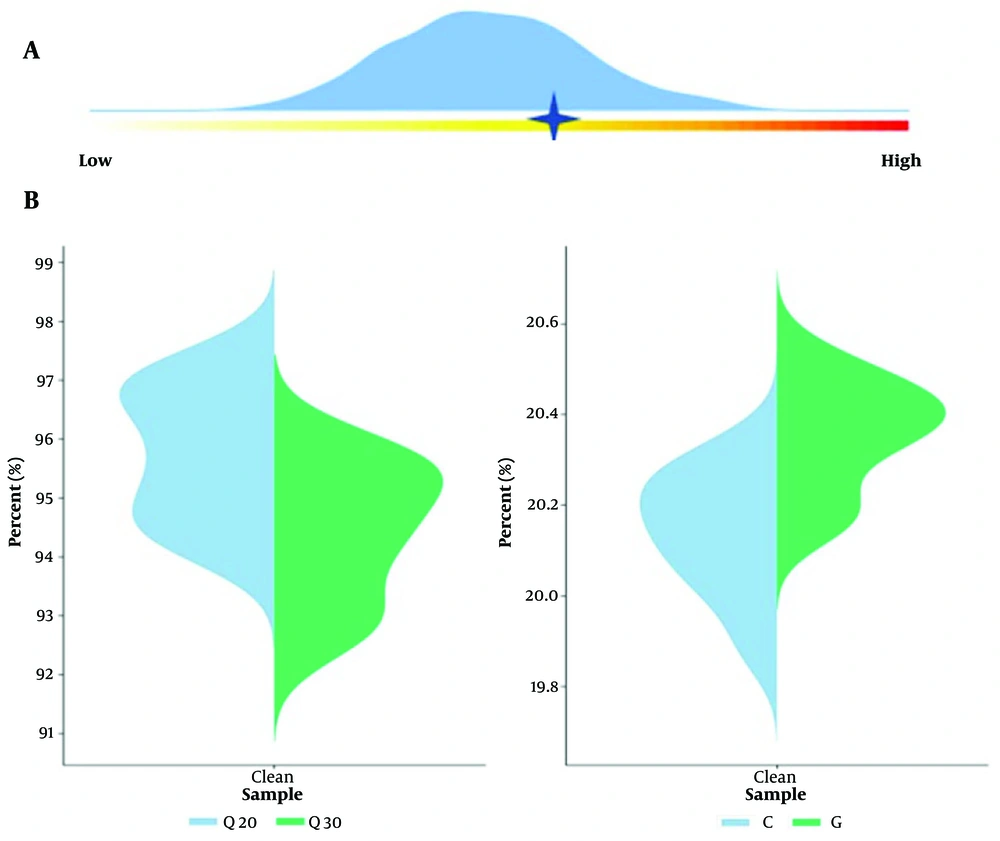
The patient's sputum was collected for pathogen examination, and no pathogen was identified by Gram staining smear microscopy. The culture result indicated a normal bacterial population, and the peripheral blood culture result was negative. The patient's bronchoalveolar lavage fluid was collected in a sterile container, sealed, and sent to a commercial laboratory (vision medicals) at low temperature for pathogen detection. Metagenomic next-generation sequencing (m-NGS) was performed, which involves high-throughput sequencing of nucleic acids extracted from biological specimens, followed by bioinformatics comparative analysis to obtain information on the microbial species and abundance in the specimens. By adding artificially synthesized tag sequences to the specimen, the proportion of human-derived nucleic acids and the content of pathogenic microorganisms in the specimen were quantitatively detected. The Human Origin Index was 29,478.64, and the pathogenic microorganism index was 17,247.72, indicating the genus Chlamydia. A total of 2,866 sequences of C. psittaci were detected, with a relative abundance of 79.53% (Figure 2).
2.3. Treatment and Outcome
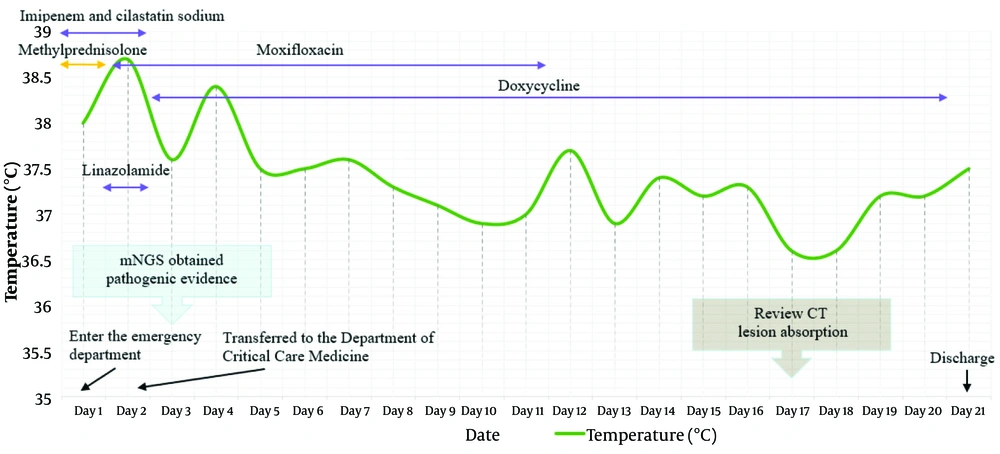
The patient's condition worsened upon admission and was sent to our emergency department. After examination, the patient's blood oxygen saturation decreased to 88%, and they were given endotracheal intubation and assisted ventilation with a ventilator. Then empirical anti-infection treatment was given with 1 g imipenem-cilastatin sodium, and 40 mg methylprednisolone was administered for anti-inflammatory treatment. The next day, the patient was transferred to the Intensive Care Unit and continued to receive an intravenous infusion of 1 g imipenem-cilastatin sodium once a day, along with 0.6 g linezolid twice a day and 250 mL moxifloxacin once a day for anti-infection treatment. On the third day, a clear pathogenic diagnosis was obtained, confirming the infection of C. psittaci. Therefore, imipenem-cilastatin sodium and linezolid were discontinued, and doxycycline 0.1 g was intravenously administered twice a day for anti-infection treatment.
On the 17th day, the patient underwent a follow-up chest CT scan, which showed pleural effusion on both sides accompanied by incomplete expansion of adjacent lung tissue. The effusion was reduced compared to the right anterior side, and the lung inflammation was absorbed more than before. After 21 days of treatment in the hospital, the patient's cough and sputum improved compared to before, there was no fever, and the condition was stable, allowing for discharge. The patient's temperature and treatment status are shown in Figure 3. After one month, the patient returned to the hospital for a follow-up examination, and all blood indicators returned to normal, with a good prognosis.
3. Discussion
Chlamydia psittaci mainly infects birds, with a global prevalence of about 20% among birds and only occasionally transmitted to humans (2, 11, 12). However, some studies have proved that horses are a new source of infection and can cause interpersonal transmission, which may underestimate the incidence rate of C. psittaci in the population (1, 13, 14). Zhejiang province in China has a humid subtropical monsoon climate, with mountains and hills accounting for 74.63% of the total area and a forest coverage rate of 60.5%, providing suitable habitats for birds (15). The patient we reported denies any contact history with birds and horses, making it difficult to trace the source of the pathogen. However, if the patient resides in rural areas for a long time, there may be a history of contact with birds and their excrement or other poultry animals that the patient was not aware of. Therefore, for pneumonia caused by unknown infections, more attention should be paid to the patient's life history. Especially for rural patients or those in special occupations such as poultry farming, the possibility of Psittacosis infection should be considered.
The symptoms of C. psittaci infection usually include fever, cough, sputum production, headache, muscle soreness, etc. Severe cases may experience breathing difficulties, such as our patient's repeated fluctuations in blood oxygen saturation upon admission and receiving ventilator-assisted ventilation. In addition, once patients experience headaches or other mental abnormalities, it is necessary to suspect the possibility of meningitis, which is one of the main causes of patient death (5, 16). Pneumonia is the most common disease caused by C. psittaci infection. On imaging, it usually presents as nodules in the upper lobe of the lungs on chest CT, accompanied by mild dizziness. There are flocculent high-density shadows in both lung lobes, and sometimes consolidation and pleural effusion can be seen (17). However, these manifestations are not specific and require differential diagnosis with Legionella, Mycoplasma, and fungal infections (7). Chest lesions can generally be absorbed 2 - 4 weeks after drug treatment (18). Although CT findings are not sufficient as a diagnostic basis, they can provide clues for diagnosis and indicate the patient's prognosis.
Generally speaking, in vitro culture is the gold standard for diagnosing C. psittaci. However, due to its infectivity and harmfulness, it needs to be cultured in a level 3 biosafety laboratory, and most hospital clinical laboratories cannot meet this standard. In addition, the cultivation of C. psittaci is also affected by sample transportation conditions and the use of antibiotics by patients. Therefore, cultivation is usually not the preferred diagnostic method (19). The detection of elevated levels of parrot fever-specific IgM antibodies in serum samples during the acute infection or recovery period of patients can effectively assist in diagnosis. However, the specificity of serological testing methods is not 100%, and antibodies may cross-react with other Chlamydia species. Due to the rarity of the disease, most hospitals do not have corresponding testing programs, which also leads to doctors ignoring suspicion of the disease in the early stages of diagnosis.
Other hematological indicators such as white blood cells, neutrophils, high-sensitivity C-reactive protein, procalcitonin, and cytokines can only assist in the diagnosis and monitoring of infection and inflammation, and do not have specificity. PCR can quickly and accurately obtain detection results, but lower respiratory tract samples need to be collected and sent for testing within 4 weeks after the onset of symptoms. Once the acute infection period is exceeded or samples are taken from the upper respiratory tract, false negative results may occur (6, 20). Currently, m-NGS has become a commonly used method for detecting infectious pathogens in clinical practice. Not only is it highly sensitive, but it can also quickly obtain detection results through direct specimen submission.
In addition, unlike PCR, m-NGS has a wide detection range and does not require doctors to suspect C. psittaci infection and prescribe corresponding testing items. It can directly detect all microbial communities from specimens and provide diagnostic recommendations based on the relative abundance of sequencing (21). The application of m-NGS in clinical testing has now become mature. In the past, due to high fees, most patients could not afford it. Now m-NGS can limit the detection range according to the detection needs, which greatly reduces the detection cost and promotes the popularization of this technology in clinical testing. Here, we quickly obtained the diagnostic evidence of C. psittaci infection in patients through m-NGS, and adjusted our antibiotic selection to timely block the further development of the disease.
The use of antibiotics needs to be adjusted according to the patient's condition. Tetracycline antibiotics are the preferred antibiotics for treating Psittacosis, such as doxycycline or minocycline. When patients are allergic to tetracycline, macrolide antibiotics such as azithromycin can be chosen. In addition, fluoroquinolone drugs such as moxifloxacin and levofloxacin have also been shown to be effective against C. psittaci (22, 23). The treatment should last for 14 days, otherwise it may cause recurrence (18). Due to the severity of the patient's condition, after obtaining a clear diagnosis, we discontinued the use of imipenem-cilastatin sodium and linezolid, and treated with intravenous infusion of doxycycline combined with moxifloxacin. After 16 days of continuous treatment, the patient's blood indicators returned to normal, and the lung imaging lesions were absorbed more than before. After discharge, the patient continued taking moxifloxacin orally for 6 days, and returned to our hospital for a follow-up examination two weeks later, which showed a good prognosis.
3.1. Conclusions
When clinical doctors encounter pneumonia caused by unknown infection and conventional antibiotic treatment is ineffective, they should increase suspicion of C. psittaci infection. Untimely diagnosis and treatment can lead to the deterioration of the disease, and even patient death. Therefore, it is necessary to pay attention to the application of m-NGS in clinical pathogen detection, shorten the detection time, and achieve early and accurate detection and treatment.